Post by dig on Mar 10, 2016 22:40:26 GMT -5
1) DPC safety:
The safety of DPC gets talked about allot. What does not get talked about is why this is a game changer for the RNAi field. To see how just how Delivery has plagued the industry Read: The Business of RNAi Therapeutics in 2012.
www.nature.com/mtna/journal/v1/n2/full/mtna20119a.html
A few quote from the Article
“most Big Pharma companies had a stake in the technology. Yet, the US$2.5B–3.5B in investments largely failed to formulate sound strategies for the real technical challenges such as delivery”
“Symbolizing the value shift from RNAi triggers to delivery, Alnylam, which once relied on its RNAi trigger IP for its industry-leading position, has been sued by Tekmira for scheming to unlawfully gain control and ownership over SNALP technology”
DPC has shown no major Adverse Effects. AE’s is what has plagued the RNAi field for years. AE’s have cause major pharmaceutical companies to discontinue their RNAi programs.
2) DPC effectiveness:
DPC has shown the Highest KD in animal models. DPC has shown the Highest KD in humans ever recorded in a single dose. What this really means is DPC is delivering the triggers to the target very well. DPC is the Safest and most Effective of the RNAi delivery methods. Arrowhead has solved the RNAi delivery problem. This alone will forever change the RNAi field.
3) ARC-520:
ARC-520 has already proven to be effective with the KD Of the entire HBV genome. Let’s look at what a single dose will do.
ir.arrowheadresearch.com/releasedetail.cfm?ReleaseID=932948
A single-dose of ARC-520 showed a mean maximal 92% (1.2 log) reduction in circulating HBeAg and a best reduction of 98% (1.7 log). Similar mean maximal reductions were also demonstrated in HBV core-related antigen (HBcrAg) from both HBeAg-negative and -positive patients. ARC-520 is designed to silence all gene products expressed by HBV cccDNA, so this data suggests that it may be substantially disrupting additional viral functions. NUC-naïve, HBeAg-positive patients, best peak HBsAg reduction has been 99% (1.9 log) and the mean maximum HBsAg reduction has been 1.05 log
Arrowhead seems to be convinced that it will take more than just HBsAg KD to “awaken” the immune system. This could set any competition in HBV back several years. HBV is a DNA virus that the pharmaceutical industry has been unable to conquer for decades. No one has been able to KD HBsAg directly, let alone HBsAg and All the other HBV antigens. My point is, ARC-520 very well may be the only way to get consistent Functional Cures for the foreseeable future. 7 of 9 chimps showing an immune response makes it very likely that ARC-520-521 will achieve FC’s in humans as a mono therapy. Now that the phase 2 studies are underway it could be literally a matter of weeks before we see the first patients to get to HBsAg negative (the first step to Functional Cure).
4) The Arrowhead team:
The chimp study started ARC-520 dosing around July 2014
The first doses of siHBV-I were given in the January 2015
In the span of 6 months the Arrowhead team:
1) Determined the KD in humans was not what they expected.
2) Determined the integrated DNA was a source of HBs-Ag.
3) Found the Trigger they needed to use to turn off the HBsAg coming from the integrated DNA.
4) Developed the drug.
5) Manufactured the drug.
6) Tested the drug.
Around April the Arrowhead team had tested what will be ARC-521 in the chimps.
Arrowhead went from the observation that they needed a drug that would KD HBsAg in the integrated DNA, to running the necessary Animal safety trials For ARC-521 in an incredibly short time frame. This removes any uncertainty that the Arrowhead team can deliver.
5) In the goals for 2015 Arrowhead revealed they would announce the first subcutaneous administration or extra-hepatic delivery preclinical programs. Arrowhead delivered both
First they announced: ARC-HIF2 that is designed to reduce the production of hypoxia-inducible factor 2 alpha, or HIF-2 alpha, to treat clear cell renal cell carcinoma. It is the first drug candidate using a new DPC™ delivery vehicle designed to target tissues outside of the liver.
Not only is it the first extra-hepatic delivery but, it is shows how effective arrowheads cancer drug’s can be by actually reducing the tumor size.” Treated mice exhibited statistically significant reductions in size and weight” link to poster files.shareholder.com/downloads/AMDA-2OTJP1/682304963x0x851963/8287350C-D9AD-4D97-990E-97E0A409B259/ECCO_2015_RCC_Poster_Final.pdf
One of the major advantages of RNAi is it effective against any Dieses. Many cancers are considered un-drugable but, all that is about to change with extra-hepatic DPC.
ARC-LPA that is designed to reduce production of apolipoprotein A, or apo(a), a key component of lipoprotein(a), or Lp little a, which has been genetically linked with increased risk of cardiovascular diseases. ARC-LPA employs Arrowhead’s new hepatic delivery format being developed for subcutaneous administration.
6) Nearly 2 billion $ in Research and IP.
Arrowhead acquired the RNAi Assets of Roche the 3rd biggest pharmaceutical company on the planet. Roche spent nearly a billion dollars developing the program and generated many patents to protect the technology. All this and the Madison facility with all its equipment and personal were assigned to Arrowhead in October 2011. Overnight this small pharmaceutical company was transformed. Arrowhead now had a state of the art facility that only big pharma would have the recourses to build. More importantly They got the People to fill it.
In March 2015 all the RNAi Assets of Novartis, the biggest pharmaceutical company on the planet were acquired by Arrowhead. ir.arrowheadresearch.com/releasedetail.cfm?ReleaseID=900109
“We anticipate this acquisition will provide us with expanded freedom to operate, proprietary technology that appears to enhance the activity of RNAi triggers”.
“We now have additional flexibility to optimize each new candidate using the most effective RNAi-trigger design and modifications”. ARC-f12 and ARC-LPA use Novartis Intellectual property for the triggers they chose, effectively leaving ALNY irrelevant to Arrowhead.
6) Big pharma has a vested interest in Arrowheads success.
Out of the top 25 pharmaceutical companies in the world Novartis ranks #1 and Roche Ranks #3
both of these companies have a vested interest in ARWR success. Novartis owns millions of shares of Arrowhead and will receive single digit royalties on any drugs that use IP acquired from Novartis.
Roche will receive single digit royalties on sales of ARC-520 and ARC-521. If Roche receives as little as 3% of sales, that would = billions over a 10 year period. It would defy logic for these companies to let Arrowhead fail over what would be chump change to either of these companies.
7) Often over looked is ARC-AAT, which is a rare genetic disorder that can lead to lung damage and liver disease. During the Phase 1 study a predetermined level of knockdown was met in healthy volunteers and transitioned the study to enroll patients with AATD. ARC-AAT was also granted orphan drug designation by the FDA in 2015. In 2016 ARC-AAT was granted EMA Orphan Drug Designation.
8) The platform:
Christopher Anzalone says it best in the 2015 q4 CC “We have always said that a key benefit of developing a suite of RNAi therapeutics is that data and experience from each program can be leveraged to inform the development of new drugs. Each of these new drugs can potentially have progressively lower risk and a faster path from discovery to human trials if they are built on an underlying delivery platform that is validated in humans. Once this validation is achieved in one candidate, it may provide better certainty in future candidates. We believe that recent data on the ARC-520 drug candidate against chronic hepatitis infection validate our DPCTM delivery system” This year we will see this in action as arrowhead executes the planned phase 1-2 for ARC-521 moves through the clinic.
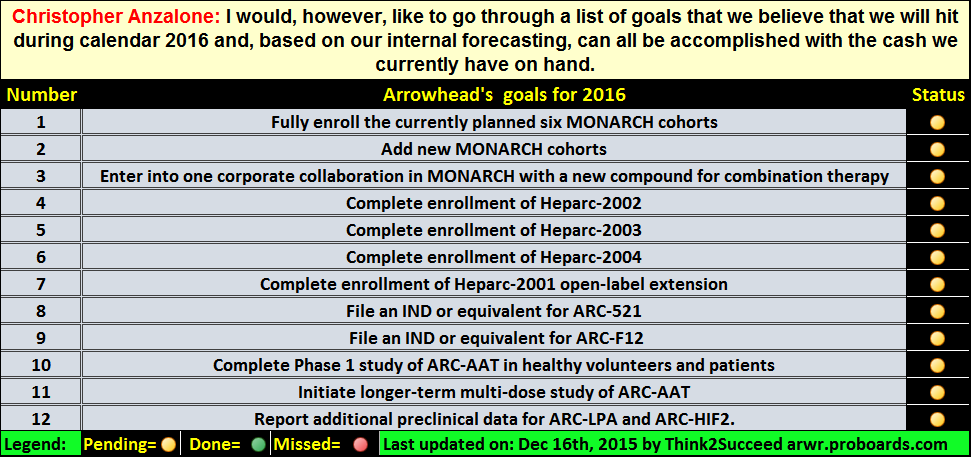
9) Lots of things will be happening in 2016: Fiscal 2015 Year End Conference Call – Prepared Remarks December 14, 2015
1. Fully enroll the currently planned 6 MONARCH cohorts
2. Add new MONARCH cohorts
3. Enter into one corporate collaboration in MONARCH with a new compound for combination therapy 4. Complete enrollment of Heparc-2002
5. Complete enrollment of Heparc-2003
6. Complete enrollment of Heparc-2004
7. Complete enrollment of Heparc-2001 open label extension
8. File IND or equivalent for ARC-521
9. File IND or equivalent for ARC-F12(an IND was pushed back to 2017 to preserve capital)
10. Complete Phase 1 study of ARC-AAT in healthy volunteers and patients
11. Initiate longer-term multi-dose study of ARC-AAT
12. Report additional preclinical data for ARC-LPA and ARC-HIF2
10) ARWR is the clear leader in HBV (a multi-$billion opportunity) and RNAi technology a (much larger than HBV opportunity). The multi-million dollar question (at least for ARWR investors) is, when will the ARWR MC accurately reflect the opportunity?
After reading the above the natural question is: if everything is so great why it is trading so cheap.
Here is the history as I see it.
Leading up to October 8th 2014 “the street” issued a “parolees reversal” or “dead cat bounce “PR at least twice a week about ARWR.
Jul 15, 2014 • Federal Reserve Chairwoman Janet Yellen declared biotech stocks over valued.
These factors had a lot to do with the short interest increasing up to the:
ARROWHEAD RESEARCH 3Q Fiscal 2014 Conference Call – Prepared Remarks August 12, 2014
Our interim results have been extremely exciting. We completed dosing of 1 and 2 mg/kg in June and though the study is still blinded, several exciting conclusions may already be drawn. We are seeing clear knockdown in both groups and duration of that knockdown has been substantially longer than we expected
For those of us that were in this stock in august of 2014 we remember how excited Management was on this day, I think for good reason we know 1) they were just getting the initial results of the 9 chimp study. 2) The patient in the placebo group just showed a sudden dramatic drop in HBsAg. We know this study was still blinded and we know that late responders showed up in nearly every cohort. If we add it all up Management would have had to think this placebo group patient was an ARC-520 patient. It was not until the data was un-blinded that Management knew the truth.
This excitement and the analysts implying a .8 log KD in the 2MG group, reversed the afterhours drop and supported the price until October 8th.
The stock sold off on the October 6th and the 7th supporting the theory “they” were going to sell on the news regardless of the Results. The fact that the KD was less that .8 log number the Analysts were implying intensified the selling.
Keeping the 3mg and 4mg number Blinded until September is the reason every event was sold into and every rally was sold into as well. “They” were waiting for the announcement that ARC-520 was a Failure.
We know the tone changed from an ARC-520 mono therapy to a Combination therapy. Giving fuel to the idea ARC-520 was a failure. In my opinion Arrowhead was questioning continuing on with ARC-520 the way they had designed the clinical studies. Here is why
We know that they had approval to begin the US study on Apr 13, 2015. The next month they started the study. The original completion date was October 2015 but, was moved to June 2016.
Jun 17, 2015 Arrowhead Receives Regulatory Clearance to Begin Heparc-2002-3
The study Start Date is not the next month but, was delayed until October 2015
My theory is between May-September Arrowhead was considering just moving forward with ARC-520 in Monarch and leaving the mono therapy testing to ARC-521. Once they got the KD they expected with ARC-520 and saw the immune response in the 7 chimps they decided a mono therapy with ARC-520 was back on the table. They moved quickly to start these studies and decided to do an open label extension study of Heparc-2001.
This takes us up to AD
Between September 17 and September 28 the IBB dropped about 20%. Arrowheads Analyst Day landed right in the middle of this decline. With the previous run-up’s failing; people saw this as an easy short. Personally I watched in disbelief as the price ticked lower. I expected the buying to overwhelm the sellers and the price to rocket higher as the shorts screamed let me out.
In summary:
The reason for the current price is
1) Expectations were 2 high before October 8th 2014.
2) Keeping the 3mg and 4mg results blinded for the better part of a year
3) Delaying the start of the multi dose studies
4) Timing of Data release
5) A 30% drop in the IBB + a 20%+ drop in the Russell 2000 = ARWR is in the most hated sector after oil. Currently many small biotech stocks are trading near cash.
The safety of DPC gets talked about allot. What does not get talked about is why this is a game changer for the RNAi field. To see how just how Delivery has plagued the industry Read: The Business of RNAi Therapeutics in 2012.
www.nature.com/mtna/journal/v1/n2/full/mtna20119a.html
A few quote from the Article
“most Big Pharma companies had a stake in the technology. Yet, the US$2.5B–3.5B in investments largely failed to formulate sound strategies for the real technical challenges such as delivery”
“Symbolizing the value shift from RNAi triggers to delivery, Alnylam, which once relied on its RNAi trigger IP for its industry-leading position, has been sued by Tekmira for scheming to unlawfully gain control and ownership over SNALP technology”
DPC has shown no major Adverse Effects. AE’s is what has plagued the RNAi field for years. AE’s have cause major pharmaceutical companies to discontinue their RNAi programs.
2) DPC effectiveness:
DPC has shown the Highest KD in animal models. DPC has shown the Highest KD in humans ever recorded in a single dose. What this really means is DPC is delivering the triggers to the target very well. DPC is the Safest and most Effective of the RNAi delivery methods. Arrowhead has solved the RNAi delivery problem. This alone will forever change the RNAi field.
3) ARC-520:
ARC-520 has already proven to be effective with the KD Of the entire HBV genome. Let’s look at what a single dose will do.
ir.arrowheadresearch.com/releasedetail.cfm?ReleaseID=932948
A single-dose of ARC-520 showed a mean maximal 92% (1.2 log) reduction in circulating HBeAg and a best reduction of 98% (1.7 log). Similar mean maximal reductions were also demonstrated in HBV core-related antigen (HBcrAg) from both HBeAg-negative and -positive patients. ARC-520 is designed to silence all gene products expressed by HBV cccDNA, so this data suggests that it may be substantially disrupting additional viral functions. NUC-naïve, HBeAg-positive patients, best peak HBsAg reduction has been 99% (1.9 log) and the mean maximum HBsAg reduction has been 1.05 log
Arrowhead seems to be convinced that it will take more than just HBsAg KD to “awaken” the immune system. This could set any competition in HBV back several years. HBV is a DNA virus that the pharmaceutical industry has been unable to conquer for decades. No one has been able to KD HBsAg directly, let alone HBsAg and All the other HBV antigens. My point is, ARC-520 very well may be the only way to get consistent Functional Cures for the foreseeable future. 7 of 9 chimps showing an immune response makes it very likely that ARC-520-521 will achieve FC’s in humans as a mono therapy. Now that the phase 2 studies are underway it could be literally a matter of weeks before we see the first patients to get to HBsAg negative (the first step to Functional Cure).
4) The Arrowhead team:
The chimp study started ARC-520 dosing around July 2014
The first doses of siHBV-I were given in the January 2015
In the span of 6 months the Arrowhead team:
1) Determined the KD in humans was not what they expected.
2) Determined the integrated DNA was a source of HBs-Ag.
3) Found the Trigger they needed to use to turn off the HBsAg coming from the integrated DNA.
4) Developed the drug.
5) Manufactured the drug.
6) Tested the drug.
Around April the Arrowhead team had tested what will be ARC-521 in the chimps.
Arrowhead went from the observation that they needed a drug that would KD HBsAg in the integrated DNA, to running the necessary Animal safety trials For ARC-521 in an incredibly short time frame. This removes any uncertainty that the Arrowhead team can deliver.
5) In the goals for 2015 Arrowhead revealed they would announce the first subcutaneous administration or extra-hepatic delivery preclinical programs. Arrowhead delivered both
First they announced: ARC-HIF2 that is designed to reduce the production of hypoxia-inducible factor 2 alpha, or HIF-2 alpha, to treat clear cell renal cell carcinoma. It is the first drug candidate using a new DPC™ delivery vehicle designed to target tissues outside of the liver.
Not only is it the first extra-hepatic delivery but, it is shows how effective arrowheads cancer drug’s can be by actually reducing the tumor size.” Treated mice exhibited statistically significant reductions in size and weight” link to poster files.shareholder.com/downloads/AMDA-2OTJP1/682304963x0x851963/8287350C-D9AD-4D97-990E-97E0A409B259/ECCO_2015_RCC_Poster_Final.pdf
One of the major advantages of RNAi is it effective against any Dieses. Many cancers are considered un-drugable but, all that is about to change with extra-hepatic DPC.
ARC-LPA that is designed to reduce production of apolipoprotein A, or apo(a), a key component of lipoprotein(a), or Lp little a, which has been genetically linked with increased risk of cardiovascular diseases. ARC-LPA employs Arrowhead’s new hepatic delivery format being developed for subcutaneous administration.
6) Nearly 2 billion $ in Research and IP.
Arrowhead acquired the RNAi Assets of Roche the 3rd biggest pharmaceutical company on the planet. Roche spent nearly a billion dollars developing the program and generated many patents to protect the technology. All this and the Madison facility with all its equipment and personal were assigned to Arrowhead in October 2011. Overnight this small pharmaceutical company was transformed. Arrowhead now had a state of the art facility that only big pharma would have the recourses to build. More importantly They got the People to fill it.
In March 2015 all the RNAi Assets of Novartis, the biggest pharmaceutical company on the planet were acquired by Arrowhead. ir.arrowheadresearch.com/releasedetail.cfm?ReleaseID=900109
“We anticipate this acquisition will provide us with expanded freedom to operate, proprietary technology that appears to enhance the activity of RNAi triggers”.
“We now have additional flexibility to optimize each new candidate using the most effective RNAi-trigger design and modifications”. ARC-f12 and ARC-LPA use Novartis Intellectual property for the triggers they chose, effectively leaving ALNY irrelevant to Arrowhead.
6) Big pharma has a vested interest in Arrowheads success.
Out of the top 25 pharmaceutical companies in the world Novartis ranks #1 and Roche Ranks #3
both of these companies have a vested interest in ARWR success. Novartis owns millions of shares of Arrowhead and will receive single digit royalties on any drugs that use IP acquired from Novartis.
Roche will receive single digit royalties on sales of ARC-520 and ARC-521. If Roche receives as little as 3% of sales, that would = billions over a 10 year period. It would defy logic for these companies to let Arrowhead fail over what would be chump change to either of these companies.
7) Often over looked is ARC-AAT, which is a rare genetic disorder that can lead to lung damage and liver disease. During the Phase 1 study a predetermined level of knockdown was met in healthy volunteers and transitioned the study to enroll patients with AATD. ARC-AAT was also granted orphan drug designation by the FDA in 2015. In 2016 ARC-AAT was granted EMA Orphan Drug Designation.
8) The platform:
Christopher Anzalone says it best in the 2015 q4 CC “We have always said that a key benefit of developing a suite of RNAi therapeutics is that data and experience from each program can be leveraged to inform the development of new drugs. Each of these new drugs can potentially have progressively lower risk and a faster path from discovery to human trials if they are built on an underlying delivery platform that is validated in humans. Once this validation is achieved in one candidate, it may provide better certainty in future candidates. We believe that recent data on the ARC-520 drug candidate against chronic hepatitis infection validate our DPCTM delivery system” This year we will see this in action as arrowhead executes the planned phase 1-2 for ARC-521 moves through the clinic.
9) Lots of things will be happening in 2016: Fiscal 2015 Year End Conference Call – Prepared Remarks December 14, 2015
1. Fully enroll the currently planned 6 MONARCH cohorts
2. Add new MONARCH cohorts
3. Enter into one corporate collaboration in MONARCH with a new compound for combination therapy 4. Complete enrollment of Heparc-2002
5. Complete enrollment of Heparc-2003
6. Complete enrollment of Heparc-2004
7. Complete enrollment of Heparc-2001 open label extension
8. File IND or equivalent for ARC-521
9. File IND or equivalent for ARC-F12(an IND was pushed back to 2017 to preserve capital)
10. Complete Phase 1 study of ARC-AAT in healthy volunteers and patients
11. Initiate longer-term multi-dose study of ARC-AAT
12. Report additional preclinical data for ARC-LPA and ARC-HIF2
10) ARWR is the clear leader in HBV (a multi-$billion opportunity) and RNAi technology a (much larger than HBV opportunity). The multi-million dollar question (at least for ARWR investors) is, when will the ARWR MC accurately reflect the opportunity?
After reading the above the natural question is: if everything is so great why it is trading so cheap.
Here is the history as I see it.
Leading up to October 8th 2014 “the street” issued a “parolees reversal” or “dead cat bounce “PR at least twice a week about ARWR.
Jul 15, 2014 • Federal Reserve Chairwoman Janet Yellen declared biotech stocks over valued.
These factors had a lot to do with the short interest increasing up to the:
ARROWHEAD RESEARCH 3Q Fiscal 2014 Conference Call – Prepared Remarks August 12, 2014
Our interim results have been extremely exciting. We completed dosing of 1 and 2 mg/kg in June and though the study is still blinded, several exciting conclusions may already be drawn. We are seeing clear knockdown in both groups and duration of that knockdown has been substantially longer than we expected
For those of us that were in this stock in august of 2014 we remember how excited Management was on this day, I think for good reason we know 1) they were just getting the initial results of the 9 chimp study. 2) The patient in the placebo group just showed a sudden dramatic drop in HBsAg. We know this study was still blinded and we know that late responders showed up in nearly every cohort. If we add it all up Management would have had to think this placebo group patient was an ARC-520 patient. It was not until the data was un-blinded that Management knew the truth.
This excitement and the analysts implying a .8 log KD in the 2MG group, reversed the afterhours drop and supported the price until October 8th.
The stock sold off on the October 6th and the 7th supporting the theory “they” were going to sell on the news regardless of the Results. The fact that the KD was less that .8 log number the Analysts were implying intensified the selling.
Keeping the 3mg and 4mg number Blinded until September is the reason every event was sold into and every rally was sold into as well. “They” were waiting for the announcement that ARC-520 was a Failure.
We know the tone changed from an ARC-520 mono therapy to a Combination therapy. Giving fuel to the idea ARC-520 was a failure. In my opinion Arrowhead was questioning continuing on with ARC-520 the way they had designed the clinical studies. Here is why
We know that they had approval to begin the US study on Apr 13, 2015. The next month they started the study. The original completion date was October 2015 but, was moved to June 2016.
Jun 17, 2015 Arrowhead Receives Regulatory Clearance to Begin Heparc-2002-3
The study Start Date is not the next month but, was delayed until October 2015
My theory is between May-September Arrowhead was considering just moving forward with ARC-520 in Monarch and leaving the mono therapy testing to ARC-521. Once they got the KD they expected with ARC-520 and saw the immune response in the 7 chimps they decided a mono therapy with ARC-520 was back on the table. They moved quickly to start these studies and decided to do an open label extension study of Heparc-2001.
This takes us up to AD
Between September 17 and September 28 the IBB dropped about 20%. Arrowheads Analyst Day landed right in the middle of this decline. With the previous run-up’s failing; people saw this as an easy short. Personally I watched in disbelief as the price ticked lower. I expected the buying to overwhelm the sellers and the price to rocket higher as the shorts screamed let me out.
In summary:
The reason for the current price is
1) Expectations were 2 high before October 8th 2014.
2) Keeping the 3mg and 4mg results blinded for the better part of a year
3) Delaying the start of the multi dose studies
4) Timing of Data release
5) A 30% drop in the IBB + a 20%+ drop in the Russell 2000 = ARWR is in the most hated sector after oil. Currently many small biotech stocks are trading near cash.