A few comments from old YMB re: Cantor-Fitz talk yesterday
Jul 14, 2016 7:59:48 GMT -5
Think2Succeed, kreyn, and 6 more like this
Post by qbeing1010 on Jul 14, 2016 7:59:48 GMT -5
jimmy_greaves_101070 :
True, marc. Only change I noticed in CA's talk was more emphasis on 520, even in the other 3 quadrants. He referred to 521 as "backup" or "in case" or some such, and more than once averred 520 would be useful in eAg- patients. Made me think they are seeing sustained KD in multi-dosing.
No matter. Marketability and premium pricing (and attracting BP) are going to depend on immune system revival (ie. NOT needing never-ending treatment, as with NUCs, even if less often), and overall value will be more than proportionally enhanced if/when ISR shown in humans, as in chimps, with 521.
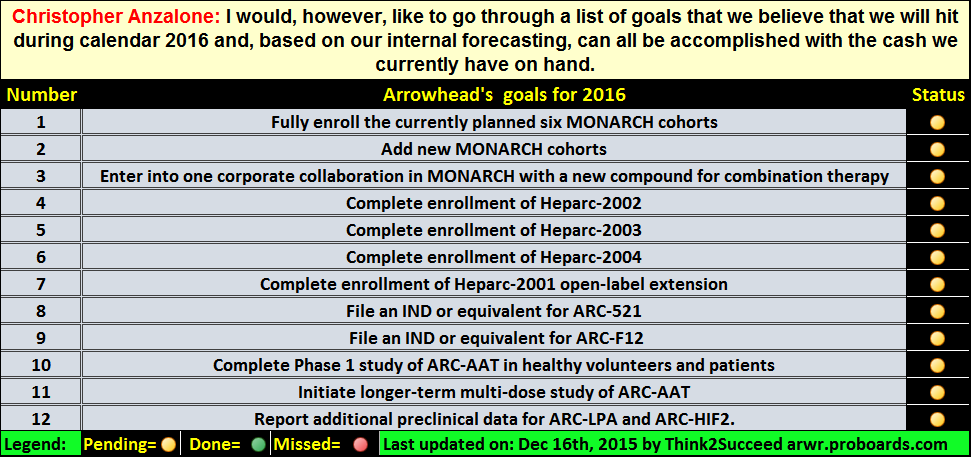
He asserted AATD readout still on target for EoY, and P2 soon.
unbiasedinvestor520:
I have seen progress here compared to master investor 2016. He almost conceded that ARC 520 doesn't only serve NN eAG+ patients as opposed to in April when it was all about them for ARC-520. And also as Jimmy already noted, ARC-521 is now not seen as the main drug. This confirms the theory that ARC-520 is performing better in other patients groups beyond NN eAg+. These are all very encouraging signs.
He is also planning ahead for 2017 when most question his ability to run the company beyond Q4 2016 due to lack of funding.
Finally, i enjoyed his confidence in DPC as the best/safest delivery despite the very high KDs shown by ALNY. I hope he is right.
It was a great talk all together in my opinion.
unbiasedinvestor520:
"The way we view FC:DNA negativity and HBsAg seroclearance"(no mention of HBsAG seroconversion)
from 2016 (ILC 2016) Dr.Walsh poster:
"Functional cure of chronic hepatitis B requires HBsAg loss and seroconversion to anti-HBs antibody"
What more?
1) No good animal models for HBV = ALNY KD in mice may not be predictive of human trials outcome
2) 520 could address the entire market.(521 viewed as an insurance)
Amazing developments in just 3 months
retired7211:
1. How did such a little company get access to so many chimps for so long when NIH was readying a cease & desist re chimp research? Dr Lanford obviously helped but who got ARWR & Lanford together? Gild, Roche, NVS?
2. CA described 521 as insurance and speculates that 520 will serve all 4 quadrants. Clearly states 520 hits all 6 HBV byproducts. Sounds good.
3. AAT phase 1 concluding soon.
hparch:
unbiased;
I wouldn't say that it's "new", just an adjustment from s-AG seroclearance to s-AG seroclearance + viral DNA clearance.
I caught this as well, and my impression is that this is more of an observational conclusion than a theoretical projection. Dr. A. talked with decisiveness about ARC-520 "crippling the life cycle" of the virus, as it almost completely eliminates 6 of the 6 genomic products it generates, in 1 large patient quadrant, and 5 of the 6 in the remaining 3/4.
He even went so far as to say that they see ARC-521 as a "back up" to ARC-520, just in case there are patients that can't be treated with ARC-520.
The underlying suggestions are profound, IMO.

dig_a_little_deeper_1:
The good news from the most recent presentation is:
Nothing has changed. They are still talking about 520 as the lead drug with 521 just in case. Still no tox issues. The tone of 520 as a monotherapy is still intact. They are still waiting on data before they do a capital raise.
Wouldn’t it be ironic if it turned out they don’t need 521 at all