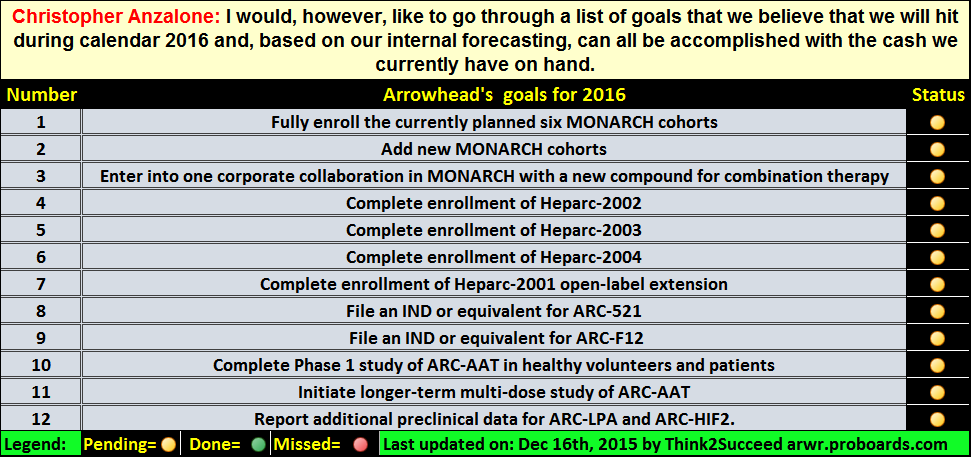
Post by digdeeper1976 on Sept 28, 2016 12:36:50 GMT -5
Acute HBV infection is characterized by the presence of HBsAg and immunoglobulin M (IgM) antibody to the core antigen, HBcAg. During the initial phase of infection, patients are also seropositive for hepatitis B e antigen (HBeAg). HBeAg is usually a marker of high levels of replication of the virus. The presence of HBeAg indicates that the blood and body fluids of the infected individual are highly contagious.
Chronic infection is characterized by the persistence of HBsAg for at least 6 months (with or without concurrent HBeAg). Persistence of HBsAg is the principal marker of risk for developing chronic liver disease and liver cancer (hepatocellular carcinoma) later in life. The likelihood that infection becomes chronic depends upon the age at which a person becomes infected. Children less than 6 years of age who become infected with the hepatitis B virus are the most likely to develop chronic infections.
80–90% of infants infected during the first year of life develop chronic infections; and
30–50% of children infected before the age of 6 years develop chronic infections.
In adults: less than 5% of otherwise healthy persons who are infected as adults will develop chronic infection; and 20–30% of adults who are chronically infected will develop cirrhosis and/or liver cancer.
HBV is responsible for 80% of primary liver cancer globally, which is almost always fatal
Treatment.
Interferon alfa (Intron A, Roferon A, Sylatron). This medicine boosts your immune system. You take it as a shot for at least 6 months. It doesn’t cure the disease. It treats liver inflammation. Long-acting interferon, peginterferon alfa2a (Pegasys, Pegasys Proclick) can also help. This drug does have side effects. It can make you feel bad all over, depressed, and zap your appetite. It also lowers your white blood cell count, which makes it harder to fight off infection.
Entecavir (Baraclude). This is the newest drug for hepatitis B. You can take it as a liquid or tablet.
Tenofovir (Viread). This drug comes as a powder or tablet. If you take it, your doctor will check often to make sure it doesn’t hurt your kidneys.
antivirals require lifelong use. Moreover, they work in only about half of the infected population, and reduce the rate of death due to liver disease by only about 40 to 70 percent.
For those who benefit from treatment, the antiviral drugs prove that medications can be effective. However, there are millions who do not benefit and are still left vulnerable. “We should not accept that a significant number of people will still die from hepatitis B-related complications despite taking the current drugs
The above was taken from various sources.
From this we can see that most of the chronically infected people were infected at birth or as children. HBV does not attack the immune system like HIV. People with HBV are not more susceptible to the flu for example. Somehow the virus keeps the immune system in check. some opinions are that HBxAg is important for the virus to continue to evade immune system control. Some believe HBcrAg is important for the viral replication. Then of course there is the HBsAg theory.
It is likely that everything the virus produces is important to It’s continued survival. Therefore a combination drug that can knockdown everything the virus produces to less than 1% of what it was, should have a devastating effect on the virus. After just a few doses of 521, a severely crippled virus will have to do battle with a fully developed (adult) immune system.
I believe Arrowhead had thought large knockdown of everything except HBsAg might be enough to get the system back in control.
This is why they decided to move forward with the 2002 and 2003 studies. This is also why they declared 521 as a backup in case large KD of HBsAg is required. I think we would have seen a Press Release if 520 would have had a high % of success in the e- NUC experienced patients. It is the NUC experienced patients that Big Pharma will be Most interested in.
I don’t believe it is all about HBsAg but, we have seen that when all of the viral products are Knocked Down (including a large HBsAg KD) it has a enormous effect on the virus. This was evident in cohort 7 with larger KD and longer duration. The next question is how much knockdown is enough. I found a study on spontaneous seroclearance of HBsAg. Here is the short version.
We studied data from 46 patients who underwent spontaneous seroclearance of HBsAg.
The combination of HBsAg level less than 200 IU/mL and a decrease of 1 or more log(10) IU/mL in a preceding 2-year period had PPVs of 97% and 100% for HBsAg seroclearance at 1 and 3 years, respectively.
Assuming that chimps are a good model for HBV, 521 should show consistent, around 3 log Knock Down. I believe this KD will apply to all groups regardless of NUC experience or HBeAg status. 3 log KD would mean that any patients with beginning HBsAg level under 5.3 log (199,526) IU/ml will be under that 200 IU/mL after 521 treatment. 5.3 log is a higher starting level than any 520 patients tested so far.
From the work Arrowhead has done with 520 we have seen lots of evidence of immune system reactivation. Clearance Profile by Dr Walsh. Presence of HBsAb in the 520 cohorts. Immune system response in the chimps. The late responders in the 520 clinical trials. Much longer duration in the cohort 7 patients. All of the above says this should work and we still have yet to see any proof. No announcement that any patients have gotten HBsAg to undetectable levels. I see a couple of possible reasons for this.
1) No patients have gotten to that Level. Again from what we have seen it appears that large KD of all that HBV produces ( including HBsAg) has the most devastating effect on the Virus. The opportunity to see that in the current studies are limited. the 4mg multiple dose cohort in Monarch. 2001 extension with 5 people from cohort 7. Single dose 6mg cohorts in the 2001 study as well. Unfortunately we do not know when these cohorts were dosed but, Arrowhead has said all cohorts should be complete before the end of 2016.
2) Arrowhead is moving 521 deeper into the clinic before they give the competition conformation of an RNAi Functional Current. What if they were seeing HBsAg getting to undetectable levels in NUC naïve e positive group? That would be great for immediate stock price. It would also give validation to the KD theory and Alnylam would be shifting into high gear on it’s clinical trials. Arrowhead has a few advantages over Alnylam.
Arrowhead was the first to pick the triggers for HBV so, they have the best ones on the planet. This should help them get the deepest KD and Duration in HBV.
(IV vs Sub-Q). The advantage of convenience goes to sub-Q. this is not as big of an advantage when you are talking about a limited number of doses for a cure. The advance of IV is you can deliver more drug and it gets to the target more quickly.
(DPC - Endosomal escape). Getting that which drives activity to target quickly (without deteriorating in the endosome) should equate to increce KD and Duration.
Endosomal escape also allows for the toxic part of the drug to leave the body quickly enhancing the safety of DPC.
(Time). Arrowhead has the lead with 520 and a smaller lead with 521. I think the safety of 520 will extend to 521. We have all ready seen this happen. Here is the phase 1 from 520.
54 subjects enrolled in 9 groups: Placebo (n=18), ARC-520 doses 0.01 mg/
kg (n=4), 0.1 mg/kg (n=4), 0.3 mg/kg (n=4), 0.6 mg/kg (n=4), 1.2 mg/kg
(n=4), 2.0 mg/kg (n=8), 3.0 mg/kg (n=4), 4 mg/kg (n=4)
And then the phase 1 for 521.
Experimental: Healthy Volunteers (NHV) Single Dose
NHV participants receiving a single dose of ARC-521 Injection at doses of 0.6, 1, 2, 4, 5 and 6 mg/kg
We have also seen this in the phase 2. Remember Arrowhead wanted to do a 2mg 4mg parallel design with 520? The US said start at 1mg and everyone else said to do a 1mg 2mg parallel study. Now Arrowhead is doing a 2mg, 4mg and 6mg multiple dose studies with 521.
This is exactly what Arrowhead Pharmaceuticals has been talking about when they say “platform play”. Once Arrowhead has proven DPC in sub-Q and extra-hepatic versions similar drugs using the same DPC will get the same leeway when designing clinical trials. The first dose of what is effective against HBsAg derived from the iDNA was given in February 2015. The fist doses of a brand new drug was given to patients September 2016. From testing an idea to a drug in phase 2 in 19 month’s is incredible. Personally I think it is amazing to watch this company in action.
Chronic infection is characterized by the persistence of HBsAg for at least 6 months (with or without concurrent HBeAg). Persistence of HBsAg is the principal marker of risk for developing chronic liver disease and liver cancer (hepatocellular carcinoma) later in life. The likelihood that infection becomes chronic depends upon the age at which a person becomes infected. Children less than 6 years of age who become infected with the hepatitis B virus are the most likely to develop chronic infections.
80–90% of infants infected during the first year of life develop chronic infections; and
30–50% of children infected before the age of 6 years develop chronic infections.
In adults: less than 5% of otherwise healthy persons who are infected as adults will develop chronic infection; and 20–30% of adults who are chronically infected will develop cirrhosis and/or liver cancer.
HBV is responsible for 80% of primary liver cancer globally, which is almost always fatal
Treatment.
Interferon alfa (Intron A, Roferon A, Sylatron). This medicine boosts your immune system. You take it as a shot for at least 6 months. It doesn’t cure the disease. It treats liver inflammation. Long-acting interferon, peginterferon alfa2a (Pegasys, Pegasys Proclick) can also help. This drug does have side effects. It can make you feel bad all over, depressed, and zap your appetite. It also lowers your white blood cell count, which makes it harder to fight off infection.
Entecavir (Baraclude). This is the newest drug for hepatitis B. You can take it as a liquid or tablet.
Tenofovir (Viread). This drug comes as a powder or tablet. If you take it, your doctor will check often to make sure it doesn’t hurt your kidneys.
antivirals require lifelong use. Moreover, they work in only about half of the infected population, and reduce the rate of death due to liver disease by only about 40 to 70 percent.
For those who benefit from treatment, the antiviral drugs prove that medications can be effective. However, there are millions who do not benefit and are still left vulnerable. “We should not accept that a significant number of people will still die from hepatitis B-related complications despite taking the current drugs
The above was taken from various sources.
From this we can see that most of the chronically infected people were infected at birth or as children. HBV does not attack the immune system like HIV. People with HBV are not more susceptible to the flu for example. Somehow the virus keeps the immune system in check. some opinions are that HBxAg is important for the virus to continue to evade immune system control. Some believe HBcrAg is important for the viral replication. Then of course there is the HBsAg theory.
It is likely that everything the virus produces is important to It’s continued survival. Therefore a combination drug that can knockdown everything the virus produces to less than 1% of what it was, should have a devastating effect on the virus. After just a few doses of 521, a severely crippled virus will have to do battle with a fully developed (adult) immune system.
I believe Arrowhead had thought large knockdown of everything except HBsAg might be enough to get the system back in control.
This is why they decided to move forward with the 2002 and 2003 studies. This is also why they declared 521 as a backup in case large KD of HBsAg is required. I think we would have seen a Press Release if 520 would have had a high % of success in the e- NUC experienced patients. It is the NUC experienced patients that Big Pharma will be Most interested in.
I don’t believe it is all about HBsAg but, we have seen that when all of the viral products are Knocked Down (including a large HBsAg KD) it has a enormous effect on the virus. This was evident in cohort 7 with larger KD and longer duration. The next question is how much knockdown is enough. I found a study on spontaneous seroclearance of HBsAg. Here is the short version.
We studied data from 46 patients who underwent spontaneous seroclearance of HBsAg.
The combination of HBsAg level less than 200 IU/mL and a decrease of 1 or more log(10) IU/mL in a preceding 2-year period had PPVs of 97% and 100% for HBsAg seroclearance at 1 and 3 years, respectively.
Assuming that chimps are a good model for HBV, 521 should show consistent, around 3 log Knock Down. I believe this KD will apply to all groups regardless of NUC experience or HBeAg status. 3 log KD would mean that any patients with beginning HBsAg level under 5.3 log (199,526) IU/ml will be under that 200 IU/mL after 521 treatment. 5.3 log is a higher starting level than any 520 patients tested so far.
From the work Arrowhead has done with 520 we have seen lots of evidence of immune system reactivation. Clearance Profile by Dr Walsh. Presence of HBsAb in the 520 cohorts. Immune system response in the chimps. The late responders in the 520 clinical trials. Much longer duration in the cohort 7 patients. All of the above says this should work and we still have yet to see any proof. No announcement that any patients have gotten HBsAg to undetectable levels. I see a couple of possible reasons for this.
1) No patients have gotten to that Level. Again from what we have seen it appears that large KD of all that HBV produces ( including HBsAg) has the most devastating effect on the Virus. The opportunity to see that in the current studies are limited. the 4mg multiple dose cohort in Monarch. 2001 extension with 5 people from cohort 7. Single dose 6mg cohorts in the 2001 study as well. Unfortunately we do not know when these cohorts were dosed but, Arrowhead has said all cohorts should be complete before the end of 2016.
2) Arrowhead is moving 521 deeper into the clinic before they give the competition conformation of an RNAi Functional Current. What if they were seeing HBsAg getting to undetectable levels in NUC naïve e positive group? That would be great for immediate stock price. It would also give validation to the KD theory and Alnylam would be shifting into high gear on it’s clinical trials. Arrowhead has a few advantages over Alnylam.
Arrowhead was the first to pick the triggers for HBV so, they have the best ones on the planet. This should help them get the deepest KD and Duration in HBV.
(IV vs Sub-Q). The advantage of convenience goes to sub-Q. this is not as big of an advantage when you are talking about a limited number of doses for a cure. The advance of IV is you can deliver more drug and it gets to the target more quickly.
(DPC - Endosomal escape). Getting that which drives activity to target quickly (without deteriorating in the endosome) should equate to increce KD and Duration.
Endosomal escape also allows for the toxic part of the drug to leave the body quickly enhancing the safety of DPC.
(Time). Arrowhead has the lead with 520 and a smaller lead with 521. I think the safety of 520 will extend to 521. We have all ready seen this happen. Here is the phase 1 from 520.
54 subjects enrolled in 9 groups: Placebo (n=18), ARC-520 doses 0.01 mg/
kg (n=4), 0.1 mg/kg (n=4), 0.3 mg/kg (n=4), 0.6 mg/kg (n=4), 1.2 mg/kg
(n=4), 2.0 mg/kg (n=8), 3.0 mg/kg (n=4), 4 mg/kg (n=4)
And then the phase 1 for 521.
Experimental: Healthy Volunteers (NHV) Single Dose
NHV participants receiving a single dose of ARC-521 Injection at doses of 0.6, 1, 2, 4, 5 and 6 mg/kg
We have also seen this in the phase 2. Remember Arrowhead wanted to do a 2mg 4mg parallel design with 520? The US said start at 1mg and everyone else said to do a 1mg 2mg parallel study. Now Arrowhead is doing a 2mg, 4mg and 6mg multiple dose studies with 521.
This is exactly what Arrowhead Pharmaceuticals has been talking about when they say “platform play”. Once Arrowhead has proven DPC in sub-Q and extra-hepatic versions similar drugs using the same DPC will get the same leeway when designing clinical trials. The first dose of what is effective against HBsAg derived from the iDNA was given in February 2015. The fist doses of a brand new drug was given to patients September 2016. From testing an idea to a drug in phase 2 in 19 month’s is incredible. Personally I think it is amazing to watch this company in action.