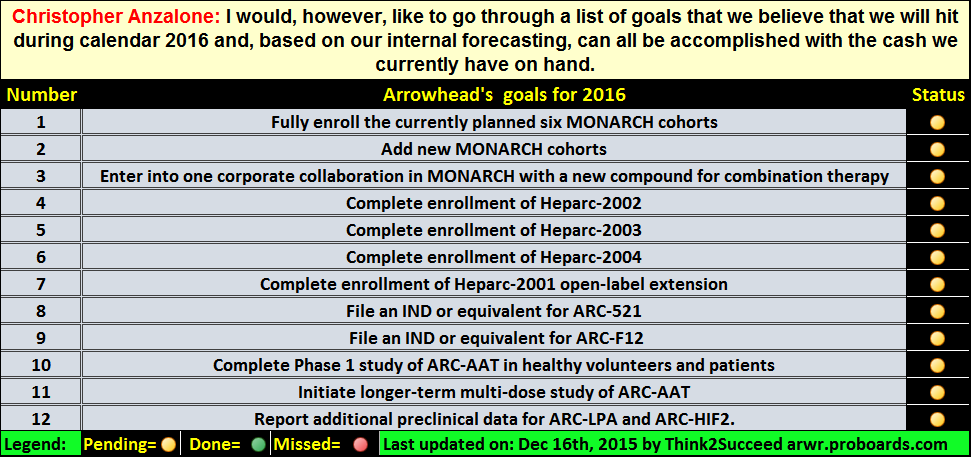
Post by dothemu on Nov 27, 2015 19:04:15 GMT -5
ARTICLE 6
EXCLUSIVE NEGOTIATION RIGHTS; RIGHTS OF FIRST NEGOTIATION; DEVELOPMENT AND COMMMERCIALIZATION PAYMENTS AND REPORTS; DILIGENCE
Section 6.01. Exclusive Negotiation Rights; Rights of First Negotiation.
(a) Prior to Initiation of a Phase 2 Clinical Trial for a given RNAi Product or Arrowhead RNAi Product directed to an Initial Target, Novartis shall have exclusive right to negotiate a license under any Intellectual Property Rights owned or exclusively licensed to Arrowhead to make, sell or otherwise commercially exploit such RNAi Product or Arrowhead RNAi Product. Arrowhead covenants and agrees that it shall not engage in any activities (a) that would interfere with Novartis’ rights under this Section 6.01 or (b) [***]. In the event that Novartis is unable to exercise its rights under this Section 6.01 as a result of such activities, Arrowhead shall pay to Novartis the sum of $[***]. Notwithstanding the foregoing payment, Novartis shall be free to pursue any and all other remedies in connection with a breach of this covenant.
(b) After Initiation of a Phase 2 Clinical Trial for a given Arrowhead RNAi Product directed to an Initial Target (each, after such Initiation, a “ROFN Candidate”), Novartis shall have a right of first negotiation (the “ROFN Rights”) if Arrowhead or any of its Affiliates proposes to Out-License any ROFN Candidate, subject to the limitations set forth in Section 6.01(f), or to enter into substantive discussions or negotiations with any Third Party relating to the Out-License of any such ROFN Candidate. Arrowhead shall give Novartis written notice thereof. Such notice shall include (i) a description in reasonable detail of the ROFN Candidate, including the status of its development and the status of any discussions with Regulatory Authorities relating thereto and (ii) the territory to which such Out-License would apply.
(c) Novartis shall have [***] days after receipt of a notice delivered pursuant to Section 6.01(b) to notify Arrowhead in writing that Novartis is interested in negotiating the applicable Out-License on commercially reasonable terms. If Novartis so notifies Arrowhead within this [***]-day period (such notice being an “Opt-in Notice”), Arrowhead shall as promptly as reasonably practicable thereafter provide Novartis with detailed information (including confidential information) regarding the applicable ROFN Candidate, including a summary of all biological, chemical and other ROFN Candidate data and an identification of all patents, Trademarks and/or other Intellectual Property Rights that are owned (wholly or partly), used or in-licensed by Arrowhead or its Affiliates and that are practiced by, arise from and/or otherwise relate to the ROFN Candidate and/or the manufacture or sale thereof (collectively, the “Data Package”). If Novartis does not timely provide an Opt-in Notice pursuant to this Section 6.01(c) with respect to a particular proposed Out-License, then, subject to Section 6.01(f), Arrowhead shall be free for a period of [***] months from the expiration of the [***]-day notice period to pursue that proposed Out-License with any Third Party. Novartis’s ROFN Rights will be deemed to have lapsed unless Arrowhead has not consummated a licensing agreement for such Data Package within such [***] months of Novartis’s decision.
(d) During the Term Sheet Period (defined below) with respect to any proposed Out-License, Arrowhead will negotiate exclusively and in good faith with Novartis regarding the applicable Out-License. During such Term Sheet Period, Arrowhead shall provide (or cause to be provided) such additional information regarding the applicable ROFN Candidate reasonably requested by Novartis that is in Arrowhead’s possession or control, provided that Arrowhead shall not be required to perform any studies or expend any material funds to generate such information. Novartis shall have up to [***] days to review such additional information. During such Term Sheet Period, Novartis may (but is not required to) submit a proposal in the form of a written term sheet with respect to the definitive terms of such Out-License, including the consideration proposed to be paid in connection with such Out-License, (including, to the extent applicable, the amount of the upfront and deferred cash consideration and, to the extent that any portion of such cash consideration is to be subject to earn out obligations, milestones or other contingencies, the nature of such contingencies, the amount and term of any royalties and the amount of any research and development funding) and other material terms and conditions of such Out-License. The “Term Sheet Period” with respect to any proposed Out-License means that number of days following Novartis’ receipt of the applicable Data Package equal to [***] days, less the number of days that elapsed between Novartis’ receipt of Arrowhead’s applicable notice delivered pursuant to Section 6.01(b) and Novartis’ delivery of the applicable Opt-In Notice.
(e) Until the expiration of the Exclusivity Period with respect to a ROFN Candidate, Arrowhead shall not (and shall cause its Affiliates not to) negotiate, discuss or enter into any agreement with any Third Party with respect to any Out-License of such ROFN Candidate. The “Exclusivity Period” with respect to any ROFN Candidate means the period beginning on the date hereof and ending (i) if Novartis has not timely delivered an Opt-In Notice in accordance with Section 6.01(c), on the [***] day following Novartis’ receipt of Arrowhead’s notice delivered pursuant Section 6.01(b), or (ii) if Novartis has timely delivered an Opt-In Notice in accordance with Section 6.01(c), then at the end of the applicable Term Sheet Period; provided that the Exclusivity Period may be extended by mutual written agreement of the Parties.
(f) Following the Exclusivity Period with respect to any ROFN Candidate, Arrowhead and its Affiliates shall be entitled to negotiate and enter into a definitive agreement with respect to an Out-License of such ROFN Candidate with any Third Party; provided that (i) if Novartis has submitted a written term sheet to Arrowhead pursuant to Section 6.01(d) during the applicable Exclusivity Period, the terms of such Out-License with any Third Party may not be less favorable, on the whole, to Arrowhead or its applicable Affiliates than the terms last proposed by Novartis in any such term sheet delivered to Arrowhead during the Exclusivity Period; (ii) such Out-License must apply to the same territory identified in the applicable notice delivered by Arrowhead to Novartis pursuant to Section 6.01(b) and not to any broader territory; and (iii) if Arrowhead does not consummate an arrangement with a Third Party with respect to such Out-License within nine months after the end of the applicable Exclusivity Period, Arrowhead’s right to negotiate with and enter into such Out-License with any Person other than Novartis shall terminate until Arrowhead has complied again with the procedures set forth in this Section 6.01.
(g) Novartis will hold, and will use Commercially Reasonable Efforts to cause its Affiliates, officers, directors, employees, accountants, counsel, consultants, advisors and agents to hold, in confidence (meaning that the applicable information will not be disclosed to any Third Party or used for any purpose other than as necessary to evaluate a potential Out-License), unless compelled to disclose by judicial or administrative process or by other requirements of Applicable Law, all confidential documents and information concerning a ROFN Candidate provided to Novartis pursuant to this Section 6.01 and Novartis shall be liable for any unauthorized disclosure or use of such confidential documents or information by its Affiliates, officers, directors, employees, accountants, counsel, consultants, advisors or agents.
Section 6.02. Royalty Payments.
(a) For sales of any RNAi Products for which Novartis or any of its Affiliates, on the one hand, and Arrowhead or any of its Affiliates, on the other hand, do not enter into an Out-License pursuant to Section 6.01, Arrowhead shall pay Novartis royalty payments at the rates set forth in the tables below with respect to each RNAi Product during the applicable Royalty Period; provided that if there is no Valid Claim Covering such RNAi Product in a given country, or if a Generic Version of the RNAi Product has been approved for commercialization in such country, then in either case the royalty payments on such RNAi Product with respect to Net Sales generated in such country shall be reduced to an amount equal to [***]% of the royalty payments otherwise due on such Net Sales generated in such country during the applicable Royalty Period. Such royalty payments shall be paid in accordance with this Section 6.02(a). For purposes of this Section 6.02(a), a “Generic Version” of a RNAi Product shall mean (i) a generic or follow-on drug product marketed in the United States that has been approved by the FDA under 21 USC § 505(j) or 21 USC § 505(b)(2); (ii) a biosimilar or interchangeable biological product marketed in the United States that has been approved by FDA under 42 USC § 262(k); and (iii) a drug or biological product marketed outside the United States for which the equivalent foreign application for approval of a generic drug or biosimilar product has been approved by the applicable Regulatory Authority in the relevant foreign jurisdiction.
(1) RNAi Products (not including RNAi Products – Alnylam Only) directed to each Initial Target
Annual Net Sales ($US)
Royalty Rates (% annual Net Sales)
Each Category 1 Target
Each Category 2 Target
<=$[***]
[***]%
[***]%
>$[***] - $[***]
[***]%
[***]%
>$[***]
[***]%
[***]%
(2) RNAi Products (not including RNAi Products – Alnylam Only) directed to each New Target
Annual Net Sales ($US)
Royalty Rate (% annual Net Sales)
<=$[***]
[***]%
>$[***] - $[***]
[***]%
>$[***]
[***]%
(3) RNAi Products – Alnylam Only directed to each Initial Target or each New Target
Annual Net Sales ($US)
Royalty Rate (% annual Net Sales)
>=$[***]
[***]%
(b) Until the termination of all applicable Royalty Periods, Arrowhead shall, within [***] days after the end of each fiscal quarter, (i) deliver to Novartis a statement setting forth in reasonable detail its calculation of Net Sales for the fiscal quarter then ended with respect to each RNAi Product that has had a First Commercial Sale, and (ii) pay Novartis any royalty amounts, as determined in accordance with this Section 6.02, that are accrued and unpaid as of the end of the fiscal quarter then ended.
(c) The “Royalty Period” with respect to any RNAi Product in a given country shall begin on the First Commercial Sale of such RNAi Product in such country and shall continue until the later of (i) the expiration of the last-to-expire Valid Claim Covering such RNAi Product in such country and (ii) eleven (11) years after the First Commercial Sale of such RNAi Product in such country.
(d) Arrowhead shall be entitled to deduct from royalty payments payable hereunder for a given RNAi Product [***]% of all Required Third Party Payments paid by Arrowhead with respect to such RNAi Product during the applicable reporting period; provided that in no event shall a deduction under this Section 6.02(d) be taken for royalty payments made with respect to such RNAi Product to [***] and its Affiliates under the [***] Agreement; and provided further that in no event shall a deduction under this Section 6.02(d) reduce any royalty payment with respect to any such RNAi Product payable by Arrowhead hereunder by more than [***]%.
Section 6.03. Milestone Payments. (a) Subject to Section 6.03(b), with respect to each RNAi Product as to which Arrowhead or any of its Affiliates, on the one hand, and Novartis or any of its Affiliates, on the other hand, have not entered into an Out-License pursuant to Section 6.01, Arrowhead shall pay Novartis the following amounts in accordance with Section 6.03(b) following the achievement of the following development and annual sales milestones (it being agreed that the following annual sales milestones will be determined on a calendar year basis):
(1)RNAi Products directed to each Initial Target
(A) Development Milestones for each Initial Target
Milestone
Payment ($US)
Each Category 1 Target
Each Category 2 Target
Initiate Phase 2 Clinical Trial
$[***]
$[***]
Initiate Phase 3 Clinical Trial
$[***]
$[***]
Obtain Regulatory Approval in the United States
$[***]
$[***]
Obtain a second Regulatory Approval
$[***]
$[***]
(B) Sales Milestones for each Initial Target
Annual Net Sales ($US)
Payment ($US)
Each Category 1 Target
Each Category 2 Target
$[***]
$[***]
$[***]
$[***]
$[***]
$[***]
$[***]
$[***]
$[***]
(2) RNAi Products directed to each New Target
(A)
Development Milestones for each New Target
Milestone
Payment ($US)
Initiate Phase 1 Clinical Trial
$[***]
Initiate Phase 2 Clinical Trial
$[***]
Initiate Phase 3 Clinical Trial
$[***]
Obtain Regulatory Approval in the United States
$[***]
Obtain a second Regulatory Approval
$[***]
(B) Sales Milestones for each New Target
Annual Net Sales ($US)
Payment ($US)
$[***]
$[***]
$[***]
$[***]
$[***]
$[***]
(b) Within [***] days after the achievement of any of the development milestones set forth in Section 6.03(a), Arrowhead shall notify Novartis thereof and shall pay the amounts due hereunder on. Within [***] days after the end of the first calendar quarter in which each sales milestone set forth in Section 6.03(a) is achieved, Arrowhead shall notify Novartis thereof and shall pay the amount due for such milestone hereunder on an RNAi Product-by-RNAi Product basis.
Section 6.04. Diligence; Development and Commercialization Reports.
(a) Diligence. Arrowhead shall use Commercially Reasonable Efforts: (i) to research and develop RNAi Products and obtain Regulatory Approvals for such RNAi Products in the RNAi Field; and (ii) to commercialize each RNAi Product in each country in which it receives Regulatory Approval.
(b) Development and Commercialization Reports. Arrowhead shall keep Novartis reasonably informed as to the progress of its and its Affiliates’ development and commercialization activities under this Agreement. Arrowhead shall provide Novartis with a written report within [***] ([***]) days after the end of each calendar year during the term of this Agreement which summaries the development and commercialization activities performed in the proceeding [***] ([***]) months, including a summary of the results of such development and commercialization activities, provided that Arrowhead shall provide written updates during such calendar year following a material change to such development and commercialization activities described in such report. Information contained in the reports as described in this Section 6.04(b) shall be considered to be Confidential Information of Arrowhead and may only be used for the purpose of confirming compliance with Section 6.04(a). Arrowhead shall promptly respond to Novartis’ reasonable questions or requests for additional clarification relating to such development and commercialization activities.
Section 6.05. Financial Records and Audit; Late Payments.
(a) Arrowhead shall, and shall cause its Affiliates and sublicensees to, maintain complete and accurate records in sufficient detail to permit Novartis to confirm the royalty and milestone payments payable under this Agreement and to verify the achievement of milestone events under this Agreement. Upon reasonable prior notice, such records shall be open during regular business hours at such place or places where such records are customarily kept for a period of three (3) years following the calendar year to which they pertain. Such inspection right shall not be exercised more often than once in any calendar year and not more frequently than once with respect to records covering any specific period of time, by an internationally recognized and independent certified public accountant selected by Novartis and reasonably acceptable to Arrowhead for the sole purpose of verifying for Novartis the accuracy of the financial reports furnished by Arrowhead, its Affiliates and sublicensees pursuant to this Agreement or of any payments made, or required to be made, by or to Novartis pursuant to this Agreement. Any such auditor shall not disclose Arrowhead’s confidential information to Novartis, except to the extent such disclosure is necessary to verify the accuracy of the financial reports furnished by Arrowhead or the amount of payments to or by Arrowhead, its Affiliates and sublicensees under this Agreement. In the event that the final result of the inspection reveals an undisputed underpayment or overpayment, the underpaid or overpaid amount shall be settled within thirty (30) days after the Parties’ receipt of such final results. Novartis shall pay for such inspections, provided, that if an underpayment of more than five percent (5%) of the amount owed hereunder for the applicable period is discovered, the fees and expenses for such inspection/audit shall be paid by Arrowhead.
(b) Arrowhead shall pay Novartis interest on any late payments under this Agreement not made within fifteen (15) days of the due date at a rate per annum equal to the lesser of the three (3) month LIBOR rate for United States Dollars plus one percent (1.0%) or the maximum applicable legal rate calculated on the total number of days payment is late.
EXCLUSIVE NEGOTIATION RIGHTS; RIGHTS OF FIRST NEGOTIATION; DEVELOPMENT AND COMMMERCIALIZATION PAYMENTS AND REPORTS; DILIGENCE
Section 6.01. Exclusive Negotiation Rights; Rights of First Negotiation.
(a) Prior to Initiation of a Phase 2 Clinical Trial for a given RNAi Product or Arrowhead RNAi Product directed to an Initial Target, Novartis shall have exclusive right to negotiate a license under any Intellectual Property Rights owned or exclusively licensed to Arrowhead to make, sell or otherwise commercially exploit such RNAi Product or Arrowhead RNAi Product. Arrowhead covenants and agrees that it shall not engage in any activities (a) that would interfere with Novartis’ rights under this Section 6.01 or (b) [***]. In the event that Novartis is unable to exercise its rights under this Section 6.01 as a result of such activities, Arrowhead shall pay to Novartis the sum of $[***]. Notwithstanding the foregoing payment, Novartis shall be free to pursue any and all other remedies in connection with a breach of this covenant.
(b) After Initiation of a Phase 2 Clinical Trial for a given Arrowhead RNAi Product directed to an Initial Target (each, after such Initiation, a “ROFN Candidate”), Novartis shall have a right of first negotiation (the “ROFN Rights”) if Arrowhead or any of its Affiliates proposes to Out-License any ROFN Candidate, subject to the limitations set forth in Section 6.01(f), or to enter into substantive discussions or negotiations with any Third Party relating to the Out-License of any such ROFN Candidate. Arrowhead shall give Novartis written notice thereof. Such notice shall include (i) a description in reasonable detail of the ROFN Candidate, including the status of its development and the status of any discussions with Regulatory Authorities relating thereto and (ii) the territory to which such Out-License would apply.
(c) Novartis shall have [***] days after receipt of a notice delivered pursuant to Section 6.01(b) to notify Arrowhead in writing that Novartis is interested in negotiating the applicable Out-License on commercially reasonable terms. If Novartis so notifies Arrowhead within this [***]-day period (such notice being an “Opt-in Notice”), Arrowhead shall as promptly as reasonably practicable thereafter provide Novartis with detailed information (including confidential information) regarding the applicable ROFN Candidate, including a summary of all biological, chemical and other ROFN Candidate data and an identification of all patents, Trademarks and/or other Intellectual Property Rights that are owned (wholly or partly), used or in-licensed by Arrowhead or its Affiliates and that are practiced by, arise from and/or otherwise relate to the ROFN Candidate and/or the manufacture or sale thereof (collectively, the “Data Package”). If Novartis does not timely provide an Opt-in Notice pursuant to this Section 6.01(c) with respect to a particular proposed Out-License, then, subject to Section 6.01(f), Arrowhead shall be free for a period of [***] months from the expiration of the [***]-day notice period to pursue that proposed Out-License with any Third Party. Novartis’s ROFN Rights will be deemed to have lapsed unless Arrowhead has not consummated a licensing agreement for such Data Package within such [***] months of Novartis’s decision.
(d) During the Term Sheet Period (defined below) with respect to any proposed Out-License, Arrowhead will negotiate exclusively and in good faith with Novartis regarding the applicable Out-License. During such Term Sheet Period, Arrowhead shall provide (or cause to be provided) such additional information regarding the applicable ROFN Candidate reasonably requested by Novartis that is in Arrowhead’s possession or control, provided that Arrowhead shall not be required to perform any studies or expend any material funds to generate such information. Novartis shall have up to [***] days to review such additional information. During such Term Sheet Period, Novartis may (but is not required to) submit a proposal in the form of a written term sheet with respect to the definitive terms of such Out-License, including the consideration proposed to be paid in connection with such Out-License, (including, to the extent applicable, the amount of the upfront and deferred cash consideration and, to the extent that any portion of such cash consideration is to be subject to earn out obligations, milestones or other contingencies, the nature of such contingencies, the amount and term of any royalties and the amount of any research and development funding) and other material terms and conditions of such Out-License. The “Term Sheet Period” with respect to any proposed Out-License means that number of days following Novartis’ receipt of the applicable Data Package equal to [***] days, less the number of days that elapsed between Novartis’ receipt of Arrowhead’s applicable notice delivered pursuant to Section 6.01(b) and Novartis’ delivery of the applicable Opt-In Notice.
(e) Until the expiration of the Exclusivity Period with respect to a ROFN Candidate, Arrowhead shall not (and shall cause its Affiliates not to) negotiate, discuss or enter into any agreement with any Third Party with respect to any Out-License of such ROFN Candidate. The “Exclusivity Period” with respect to any ROFN Candidate means the period beginning on the date hereof and ending (i) if Novartis has not timely delivered an Opt-In Notice in accordance with Section 6.01(c), on the [***] day following Novartis’ receipt of Arrowhead’s notice delivered pursuant Section 6.01(b), or (ii) if Novartis has timely delivered an Opt-In Notice in accordance with Section 6.01(c), then at the end of the applicable Term Sheet Period; provided that the Exclusivity Period may be extended by mutual written agreement of the Parties.
(f) Following the Exclusivity Period with respect to any ROFN Candidate, Arrowhead and its Affiliates shall be entitled to negotiate and enter into a definitive agreement with respect to an Out-License of such ROFN Candidate with any Third Party; provided that (i) if Novartis has submitted a written term sheet to Arrowhead pursuant to Section 6.01(d) during the applicable Exclusivity Period, the terms of such Out-License with any Third Party may not be less favorable, on the whole, to Arrowhead or its applicable Affiliates than the terms last proposed by Novartis in any such term sheet delivered to Arrowhead during the Exclusivity Period; (ii) such Out-License must apply to the same territory identified in the applicable notice delivered by Arrowhead to Novartis pursuant to Section 6.01(b) and not to any broader territory; and (iii) if Arrowhead does not consummate an arrangement with a Third Party with respect to such Out-License within nine months after the end of the applicable Exclusivity Period, Arrowhead’s right to negotiate with and enter into such Out-License with any Person other than Novartis shall terminate until Arrowhead has complied again with the procedures set forth in this Section 6.01.
(g) Novartis will hold, and will use Commercially Reasonable Efforts to cause its Affiliates, officers, directors, employees, accountants, counsel, consultants, advisors and agents to hold, in confidence (meaning that the applicable information will not be disclosed to any Third Party or used for any purpose other than as necessary to evaluate a potential Out-License), unless compelled to disclose by judicial or administrative process or by other requirements of Applicable Law, all confidential documents and information concerning a ROFN Candidate provided to Novartis pursuant to this Section 6.01 and Novartis shall be liable for any unauthorized disclosure or use of such confidential documents or information by its Affiliates, officers, directors, employees, accountants, counsel, consultants, advisors or agents.
Section 6.02. Royalty Payments.
(a) For sales of any RNAi Products for which Novartis or any of its Affiliates, on the one hand, and Arrowhead or any of its Affiliates, on the other hand, do not enter into an Out-License pursuant to Section 6.01, Arrowhead shall pay Novartis royalty payments at the rates set forth in the tables below with respect to each RNAi Product during the applicable Royalty Period; provided that if there is no Valid Claim Covering such RNAi Product in a given country, or if a Generic Version of the RNAi Product has been approved for commercialization in such country, then in either case the royalty payments on such RNAi Product with respect to Net Sales generated in such country shall be reduced to an amount equal to [***]% of the royalty payments otherwise due on such Net Sales generated in such country during the applicable Royalty Period. Such royalty payments shall be paid in accordance with this Section 6.02(a). For purposes of this Section 6.02(a), a “Generic Version” of a RNAi Product shall mean (i) a generic or follow-on drug product marketed in the United States that has been approved by the FDA under 21 USC § 505(j) or 21 USC § 505(b)(2); (ii) a biosimilar or interchangeable biological product marketed in the United States that has been approved by FDA under 42 USC § 262(k); and (iii) a drug or biological product marketed outside the United States for which the equivalent foreign application for approval of a generic drug or biosimilar product has been approved by the applicable Regulatory Authority in the relevant foreign jurisdiction.
(1) RNAi Products (not including RNAi Products – Alnylam Only) directed to each Initial Target
Annual Net Sales ($US)
Royalty Rates (% annual Net Sales)
Each Category 1 Target
Each Category 2 Target
<=$[***]
[***]%
[***]%
>$[***] - $[***]
[***]%
[***]%
>$[***]
[***]%
[***]%
(2) RNAi Products (not including RNAi Products – Alnylam Only) directed to each New Target
Annual Net Sales ($US)
Royalty Rate (% annual Net Sales)
<=$[***]
[***]%
>$[***] - $[***]
[***]%
>$[***]
[***]%
(3) RNAi Products – Alnylam Only directed to each Initial Target or each New Target
Annual Net Sales ($US)
Royalty Rate (% annual Net Sales)
>=$[***]
[***]%
(b) Until the termination of all applicable Royalty Periods, Arrowhead shall, within [***] days after the end of each fiscal quarter, (i) deliver to Novartis a statement setting forth in reasonable detail its calculation of Net Sales for the fiscal quarter then ended with respect to each RNAi Product that has had a First Commercial Sale, and (ii) pay Novartis any royalty amounts, as determined in accordance with this Section 6.02, that are accrued and unpaid as of the end of the fiscal quarter then ended.
(c) The “Royalty Period” with respect to any RNAi Product in a given country shall begin on the First Commercial Sale of such RNAi Product in such country and shall continue until the later of (i) the expiration of the last-to-expire Valid Claim Covering such RNAi Product in such country and (ii) eleven (11) years after the First Commercial Sale of such RNAi Product in such country.
(d) Arrowhead shall be entitled to deduct from royalty payments payable hereunder for a given RNAi Product [***]% of all Required Third Party Payments paid by Arrowhead with respect to such RNAi Product during the applicable reporting period; provided that in no event shall a deduction under this Section 6.02(d) be taken for royalty payments made with respect to such RNAi Product to [***] and its Affiliates under the [***] Agreement; and provided further that in no event shall a deduction under this Section 6.02(d) reduce any royalty payment with respect to any such RNAi Product payable by Arrowhead hereunder by more than [***]%.
Section 6.03. Milestone Payments. (a) Subject to Section 6.03(b), with respect to each RNAi Product as to which Arrowhead or any of its Affiliates, on the one hand, and Novartis or any of its Affiliates, on the other hand, have not entered into an Out-License pursuant to Section 6.01, Arrowhead shall pay Novartis the following amounts in accordance with Section 6.03(b) following the achievement of the following development and annual sales milestones (it being agreed that the following annual sales milestones will be determined on a calendar year basis):
(1)RNAi Products directed to each Initial Target
(A) Development Milestones for each Initial Target
Milestone
Payment ($US)
Each Category 1 Target
Each Category 2 Target
Initiate Phase 2 Clinical Trial
$[***]
$[***]
Initiate Phase 3 Clinical Trial
$[***]
$[***]
Obtain Regulatory Approval in the United States
$[***]
$[***]
Obtain a second Regulatory Approval
$[***]
$[***]
(B) Sales Milestones for each Initial Target
Annual Net Sales ($US)
Payment ($US)
Each Category 1 Target
Each Category 2 Target
$[***]
$[***]
$[***]
$[***]
$[***]
$[***]
$[***]
$[***]
$[***]
(2) RNAi Products directed to each New Target
(A)
Development Milestones for each New Target
Milestone
Payment ($US)
Initiate Phase 1 Clinical Trial
$[***]
Initiate Phase 2 Clinical Trial
$[***]
Initiate Phase 3 Clinical Trial
$[***]
Obtain Regulatory Approval in the United States
$[***]
Obtain a second Regulatory Approval
$[***]
(B) Sales Milestones for each New Target
Annual Net Sales ($US)
Payment ($US)
$[***]
$[***]
$[***]
$[***]
$[***]
$[***]
(b) Within [***] days after the achievement of any of the development milestones set forth in Section 6.03(a), Arrowhead shall notify Novartis thereof and shall pay the amounts due hereunder on. Within [***] days after the end of the first calendar quarter in which each sales milestone set forth in Section 6.03(a) is achieved, Arrowhead shall notify Novartis thereof and shall pay the amount due for such milestone hereunder on an RNAi Product-by-RNAi Product basis.
Section 6.04. Diligence; Development and Commercialization Reports.
(a) Diligence. Arrowhead shall use Commercially Reasonable Efforts: (i) to research and develop RNAi Products and obtain Regulatory Approvals for such RNAi Products in the RNAi Field; and (ii) to commercialize each RNAi Product in each country in which it receives Regulatory Approval.
(b) Development and Commercialization Reports. Arrowhead shall keep Novartis reasonably informed as to the progress of its and its Affiliates’ development and commercialization activities under this Agreement. Arrowhead shall provide Novartis with a written report within [***] ([***]) days after the end of each calendar year during the term of this Agreement which summaries the development and commercialization activities performed in the proceeding [***] ([***]) months, including a summary of the results of such development and commercialization activities, provided that Arrowhead shall provide written updates during such calendar year following a material change to such development and commercialization activities described in such report. Information contained in the reports as described in this Section 6.04(b) shall be considered to be Confidential Information of Arrowhead and may only be used for the purpose of confirming compliance with Section 6.04(a). Arrowhead shall promptly respond to Novartis’ reasonable questions or requests for additional clarification relating to such development and commercialization activities.
Section 6.05. Financial Records and Audit; Late Payments.
(a) Arrowhead shall, and shall cause its Affiliates and sublicensees to, maintain complete and accurate records in sufficient detail to permit Novartis to confirm the royalty and milestone payments payable under this Agreement and to verify the achievement of milestone events under this Agreement. Upon reasonable prior notice, such records shall be open during regular business hours at such place or places where such records are customarily kept for a period of three (3) years following the calendar year to which they pertain. Such inspection right shall not be exercised more often than once in any calendar year and not more frequently than once with respect to records covering any specific period of time, by an internationally recognized and independent certified public accountant selected by Novartis and reasonably acceptable to Arrowhead for the sole purpose of verifying for Novartis the accuracy of the financial reports furnished by Arrowhead, its Affiliates and sublicensees pursuant to this Agreement or of any payments made, or required to be made, by or to Novartis pursuant to this Agreement. Any such auditor shall not disclose Arrowhead’s confidential information to Novartis, except to the extent such disclosure is necessary to verify the accuracy of the financial reports furnished by Arrowhead or the amount of payments to or by Arrowhead, its Affiliates and sublicensees under this Agreement. In the event that the final result of the inspection reveals an undisputed underpayment or overpayment, the underpaid or overpaid amount shall be settled within thirty (30) days after the Parties’ receipt of such final results. Novartis shall pay for such inspections, provided, that if an underpayment of more than five percent (5%) of the amount owed hereunder for the applicable period is discovered, the fees and expenses for such inspection/audit shall be paid by Arrowhead.
(b) Arrowhead shall pay Novartis interest on any late payments under this Agreement not made within fifteen (15) days of the due date at a rate per annum equal to the lesser of the three (3) month LIBOR rate for United States Dollars plus one percent (1.0%) or the maximum applicable legal rate calculated on the total number of days payment is late.