More details of the Arrowhead-Roche transaction
Nov 28, 2015 12:40:57 GMT -5
bnmchoi, riche, and 1 more like this
Post by Think2Succeed on Nov 28, 2015 12:40:57 GMT -5
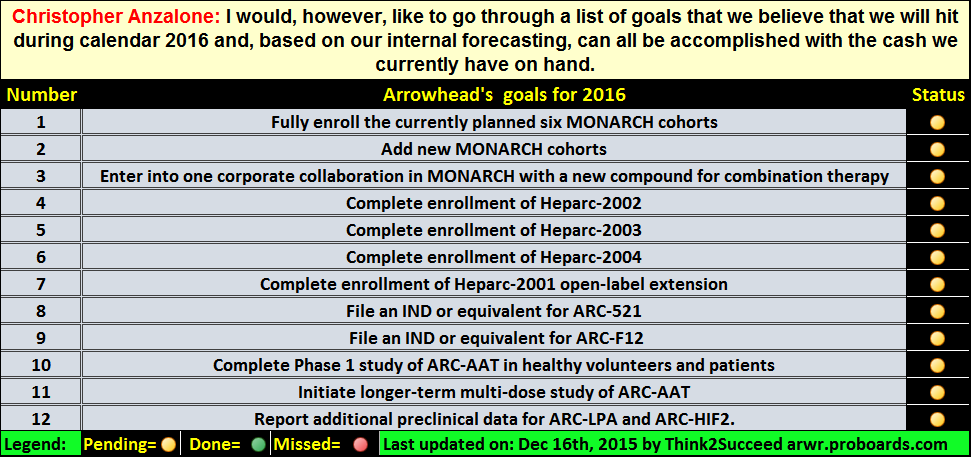
This was posted by Arrowhead around the time the deal was made but since some time has passed since then, I thought it is worth posting it for the new investors:
Arrowhead Acquisition of RNA Assets and Site
Frequently Asked Questions
1. What did Arrowhead acquire?
Arrowhead has acquired all of Roche’s current RNA therapeutics assets and its facilities in Madison, Wisconsin. Roche assembled the infrastructure, technologies, IP,and team it needed to become a leader in RNAi in a way that only a large pharmaceutical company with substantial financial resources can. Specifically Arrowhead has acquired:
-Roche Madison Inc. (formerly Mirus Bio Corporation, acquired by Roche in 2008 for $125 million), providing an advanced proprietary
RNAi delivery platform known as Dynamic PolyCunjugates™ (DPCs);
-License from Tekmira Pharmaceuticals Corp. for proprietary Stable Nucleic Acid Lipid Particles (SNALP) RNAi delivery;
-Proprietary Liposomal Nanoparticle (LNP) RNAi delivery system developed by Roche;
-$331 million license from Alnylam providing access to Alnylam’s RNAi IP and Canonical siRNA structures;
-License from City of Hope providing access to Dicer substrate siRNA structures;
-License from MDRNA (now Marina Biotech) providing access to Meroduplex siRNA structures;
-A team of over 40 leading scientists in the RNAi field and state-of-the-art facilities and infrastructure in Madison, WI.
2. How much did Arrowhead pay for the Roche RNAi Therapeutics business?
Arrowhead acquired Roche’s RNAi Therapeutics group, including its R&D center in
Madison, personnel, and comprehensive technology IP portfolio. Roche received an
equity stake in Arrowhead of just under 10% in restricted common stock. Roche was
also given limited rights to negotiate for a defined number of products and will
receive late-stage milestone payments, which only trigger after drug regulatory
approval, and customary low single digit royalty payments based on sales on certain
products.
3. What is the estimated value of the business and assets acquired?
Roche invested substantial capital to build its RNAi program. For instance, it paid
$125 million to acquire Mirus in 2008, and under the alliance agreement entered into
with Alnylam in 2007, it paid $331 million in upfront payments and an equity
investment. Additionally Roche has incurred costs for other milestones, licences,
collaboration payments and development costs. Arrowhead is fortunate to stand on these broad shoulders.
These assets are of great value to the Company because of
synergies with our current RNAi technologies and how they will position us going
forward. We believe that this acquisition positions the Company as a clear leader in
delivery, which remains the great limiting factor in RNAi therapeutics. Arrowhead
now has access to what we believe are five of the most technologically advanced small
RNA delivery systems in the world. Beyond delivery, the company has acquired
comprehensive R&D capabilities and an intellectual property framework on which to
build diverse RNAi therapeutics. Through the RNAi licenses Arrowhead has acquired,
the Company now has broad freedom to operate within the three primary siRNA
formats. This provides us with the ability to optimize not only the delivery system by
target, disease state, and organ system; but also the RNAi chemistry and siRNA
structure. No other company has this type of flexibility to optimize RNAi therapeutics.
Therefore, Arrowhead has substantial tools with which to build its own therapeutic
pipeline, and this strengthens our partnering capabilities and places the company on a
path toward financial self-sufficiency.
4. Were there other parties bidding on the assets?
Yes
5. Why did Arrowhead acquire these assets and operations?
Arrowhead management viewed this as too big an opportunity to pass up. It would
have been extremely difficult for any small biotech company to do what Roche did: it
invested a very large amount of capital to systematically acquire all resources needed
to build a pipeline of diverse RNAi therapeutics, from sequence to delivery. Roche
then did not rush to the clinic, but rather spent time and resources developing and
understanding its technologies. Therefore, what Arrowhead was able to acquire was
a relatively mature and complete set of capabilities.
This acquisition is of significant strategic value based on the assets and the Madison
team alone, and also provides accretive value when combining them with
Arrowhead’s existing RNAi assets. Arrowhead becomes a comprehensive RNAi drug
company with multiple leading delivery solutions, access to broad RNAi IP, and a
powerful engine for developing therapeutics. These can be used to build an internal
pipeline of drug candidates and to enter into partnerships with potentially significant
economics associated with them. The latter is an important part of the Company’s
capital strategy and the new assets and capabilities greatly enhance Arrowhead’s
value as a potential partner. The Company’s model is to leverage its now substantial
RNAi estate to secure partnerships that will pay for operations and drug development.
6. Will Arrowhead still seek to partner its RNAi programs?
Yes. Arrowhead’s strategy will be to leverage its robust RNAi portfolio and
development capabilities to attract collaborations with pharmaceutical and biotech
companies. The Company believes its partnering capabilities have been enhanced
based on the strength of its RNAi portfolio and newfound R&D facilities, both of which
provide a foundation for achieving financial self sufficiency and increased shareholder
value.
The combined new operation addresses what Arrowhead sees as an evolving need
from Pharma for a full-solution, specialized RNAi partner. Arrowhead believes the
transaction strengthens its partnering power as it can now offer potential partners a
comprehensive solution with the following capabilities:
-Broad IP estate and technology licenses across several disease areas and
indications;
-Multiple formats/strategies to engage RNAi mechanism;
-Multiple delivery platforms;
-State-of-the-art specialized facilities; and
-World class expertise and experience from target discovery stage through clinical
trials.
7. Has this transaction changed Arrowhead’s strategic initiatives?
No. What has changed is the addition of R&D facilities making Arrowhead a
comprehensive drug development company with in-house development resources.
With this transaction, the Company gains more robust RNAi competencies that it can
leverage, along with its new R&D capabilities to progress its RNAi and non-RNAi
technologies and attract collaborations.
8. Will Arrowhead continue to advance its non-RNAi divisions?
Arrowhead’s commercialization strategy continues to include internal development
and collaborations for its RNAi and non-RNAi programs. Arrowhead believes that
Roche’s veteran teams substantially increase its value and capabilities in both of these
areas. With the state-of-the-art R&D infrastructure acquired in this transaction,
Arrowhead plans to leverage these competencies for development of all of its
programs.
9. How will Arrowhead fund its new operations?
Arrowhead will fund its operations through a strategic combination of in-house
development, partnerships and licensing agreements. Approximately $6 million was
raised through a recently announced private placement for continuing operations,
and concurrent with the Roche transaction, Arrowhead announced that it had raised
an additional $4 million to fund initial operations at the new facility. The Company
also entered into an agreement for up to $15 million in future funding from Lincoln
Park Capital to be drawn if and when needed. As mentioned, it is our plan and priority to
leverage partnership opportunities to bring in potentially substantial nondilutive
capital to fund operations of our drug pipeline.
10. How do these platforms differ from the current Calando delivery platform?
Calando’s technology is the only clinically validated means of delivering siRNA to
organs other than the liver, and this is an important advantage. Arrowhead believes
that the DPC, SNALP, and LNP technologies from Roche each have their own strengths
in liver and non-liver delivery, and animal data Roche has generated suggest that the
systems are also extremely safe. In addition, Arrowhead intends to explore the
application of Leonardo Biosystem’s multistage delivery platform combined with the
Roche delivery technologies to make them even more efficient. This acquisition
consolidates five of the most advanced nucleic acid delivery platforms as well as
proprietary cellular targeting systems with the combined ability to reach a wide range
of disease types and sites. One of the most attractive aspects of this acquisition is the
integration of Arrowhead’s technology into what Roche has built. Arrowhead
understands that no single delivery platform will be optimal for all targets. With the
addition of Roche’s delivery platforms, Arrowhead is now able to offer the most
comprehensive solution to Pharma partners and collaborators that want to target the
disease types that fit with their own internal pipeline strategy.
11. What data has been presented on the technology/IP?
Roche’s technology and developments have been closely held. Arrowhead will work
with the team in Madison to publish select data as appropriate, and provide investors
access to data previously published.
12. What is the expected timing for progressing these technologies?
Arrowhead will pursue clinical paths and partnerships to further advance these
technologies. The Company’s roadmap for commercialization and will provide
additional detail on initial areas of focus at a later time.
T2S
Arrowhead Acquisition of RNA Assets and Site
Frequently Asked Questions
1. What did Arrowhead acquire?
Arrowhead has acquired all of Roche’s current RNA therapeutics assets and its facilities in Madison, Wisconsin. Roche assembled the infrastructure, technologies, IP,and team it needed to become a leader in RNAi in a way that only a large pharmaceutical company with substantial financial resources can. Specifically Arrowhead has acquired:
-Roche Madison Inc. (formerly Mirus Bio Corporation, acquired by Roche in 2008 for $125 million), providing an advanced proprietary
RNAi delivery platform known as Dynamic PolyCunjugates™ (DPCs);
-License from Tekmira Pharmaceuticals Corp. for proprietary Stable Nucleic Acid Lipid Particles (SNALP) RNAi delivery;
-Proprietary Liposomal Nanoparticle (LNP) RNAi delivery system developed by Roche;
-$331 million license from Alnylam providing access to Alnylam’s RNAi IP and Canonical siRNA structures;
-License from City of Hope providing access to Dicer substrate siRNA structures;
-License from MDRNA (now Marina Biotech) providing access to Meroduplex siRNA structures;
-A team of over 40 leading scientists in the RNAi field and state-of-the-art facilities and infrastructure in Madison, WI.
2. How much did Arrowhead pay for the Roche RNAi Therapeutics business?
Arrowhead acquired Roche’s RNAi Therapeutics group, including its R&D center in
Madison, personnel, and comprehensive technology IP portfolio. Roche received an
equity stake in Arrowhead of just under 10% in restricted common stock. Roche was
also given limited rights to negotiate for a defined number of products and will
receive late-stage milestone payments, which only trigger after drug regulatory
approval, and customary low single digit royalty payments based on sales on certain
products.
3. What is the estimated value of the business and assets acquired?
Roche invested substantial capital to build its RNAi program. For instance, it paid
$125 million to acquire Mirus in 2008, and under the alliance agreement entered into
with Alnylam in 2007, it paid $331 million in upfront payments and an equity
investment. Additionally Roche has incurred costs for other milestones, licences,
collaboration payments and development costs. Arrowhead is fortunate to stand on these broad shoulders.
These assets are of great value to the Company because of
synergies with our current RNAi technologies and how they will position us going
forward. We believe that this acquisition positions the Company as a clear leader in
delivery, which remains the great limiting factor in RNAi therapeutics. Arrowhead
now has access to what we believe are five of the most technologically advanced small
RNA delivery systems in the world. Beyond delivery, the company has acquired
comprehensive R&D capabilities and an intellectual property framework on which to
build diverse RNAi therapeutics. Through the RNAi licenses Arrowhead has acquired,
the Company now has broad freedom to operate within the three primary siRNA
formats. This provides us with the ability to optimize not only the delivery system by
target, disease state, and organ system; but also the RNAi chemistry and siRNA
structure. No other company has this type of flexibility to optimize RNAi therapeutics.
Therefore, Arrowhead has substantial tools with which to build its own therapeutic
pipeline, and this strengthens our partnering capabilities and places the company on a
path toward financial self-sufficiency.
4. Were there other parties bidding on the assets?
Yes
5. Why did Arrowhead acquire these assets and operations?
Arrowhead management viewed this as too big an opportunity to pass up. It would
have been extremely difficult for any small biotech company to do what Roche did: it
invested a very large amount of capital to systematically acquire all resources needed
to build a pipeline of diverse RNAi therapeutics, from sequence to delivery. Roche
then did not rush to the clinic, but rather spent time and resources developing and
understanding its technologies. Therefore, what Arrowhead was able to acquire was
a relatively mature and complete set of capabilities.
This acquisition is of significant strategic value based on the assets and the Madison
team alone, and also provides accretive value when combining them with
Arrowhead’s existing RNAi assets. Arrowhead becomes a comprehensive RNAi drug
company with multiple leading delivery solutions, access to broad RNAi IP, and a
powerful engine for developing therapeutics. These can be used to build an internal
pipeline of drug candidates and to enter into partnerships with potentially significant
economics associated with them. The latter is an important part of the Company’s
capital strategy and the new assets and capabilities greatly enhance Arrowhead’s
value as a potential partner. The Company’s model is to leverage its now substantial
RNAi estate to secure partnerships that will pay for operations and drug development.
6. Will Arrowhead still seek to partner its RNAi programs?
Yes. Arrowhead’s strategy will be to leverage its robust RNAi portfolio and
development capabilities to attract collaborations with pharmaceutical and biotech
companies. The Company believes its partnering capabilities have been enhanced
based on the strength of its RNAi portfolio and newfound R&D facilities, both of which
provide a foundation for achieving financial self sufficiency and increased shareholder
value.
The combined new operation addresses what Arrowhead sees as an evolving need
from Pharma for a full-solution, specialized RNAi partner. Arrowhead believes the
transaction strengthens its partnering power as it can now offer potential partners a
comprehensive solution with the following capabilities:
-Broad IP estate and technology licenses across several disease areas and
indications;
-Multiple formats/strategies to engage RNAi mechanism;
-Multiple delivery platforms;
-State-of-the-art specialized facilities; and
-World class expertise and experience from target discovery stage through clinical
trials.
7. Has this transaction changed Arrowhead’s strategic initiatives?
No. What has changed is the addition of R&D facilities making Arrowhead a
comprehensive drug development company with in-house development resources.
With this transaction, the Company gains more robust RNAi competencies that it can
leverage, along with its new R&D capabilities to progress its RNAi and non-RNAi
technologies and attract collaborations.
8. Will Arrowhead continue to advance its non-RNAi divisions?
Arrowhead’s commercialization strategy continues to include internal development
and collaborations for its RNAi and non-RNAi programs. Arrowhead believes that
Roche’s veteran teams substantially increase its value and capabilities in both of these
areas. With the state-of-the-art R&D infrastructure acquired in this transaction,
Arrowhead plans to leverage these competencies for development of all of its
programs.
9. How will Arrowhead fund its new operations?
Arrowhead will fund its operations through a strategic combination of in-house
development, partnerships and licensing agreements. Approximately $6 million was
raised through a recently announced private placement for continuing operations,
and concurrent with the Roche transaction, Arrowhead announced that it had raised
an additional $4 million to fund initial operations at the new facility. The Company
also entered into an agreement for up to $15 million in future funding from Lincoln
Park Capital to be drawn if and when needed. As mentioned, it is our plan and priority to
leverage partnership opportunities to bring in potentially substantial nondilutive
capital to fund operations of our drug pipeline.
10. How do these platforms differ from the current Calando delivery platform?
Calando’s technology is the only clinically validated means of delivering siRNA to
organs other than the liver, and this is an important advantage. Arrowhead believes
that the DPC, SNALP, and LNP technologies from Roche each have their own strengths
in liver and non-liver delivery, and animal data Roche has generated suggest that the
systems are also extremely safe. In addition, Arrowhead intends to explore the
application of Leonardo Biosystem’s multistage delivery platform combined with the
Roche delivery technologies to make them even more efficient. This acquisition
consolidates five of the most advanced nucleic acid delivery platforms as well as
proprietary cellular targeting systems with the combined ability to reach a wide range
of disease types and sites. One of the most attractive aspects of this acquisition is the
integration of Arrowhead’s technology into what Roche has built. Arrowhead
understands that no single delivery platform will be optimal for all targets. With the
addition of Roche’s delivery platforms, Arrowhead is now able to offer the most
comprehensive solution to Pharma partners and collaborators that want to target the
disease types that fit with their own internal pipeline strategy.
11. What data has been presented on the technology/IP?
Roche’s technology and developments have been closely held. Arrowhead will work
with the team in Madison to publish select data as appropriate, and provide investors
access to data previously published.
12. What is the expected timing for progressing these technologies?
Arrowhead will pursue clinical paths and partnerships to further advance these
technologies. The Company’s roadmap for commercialization and will provide
additional detail on initial areas of focus at a later time.
T2S