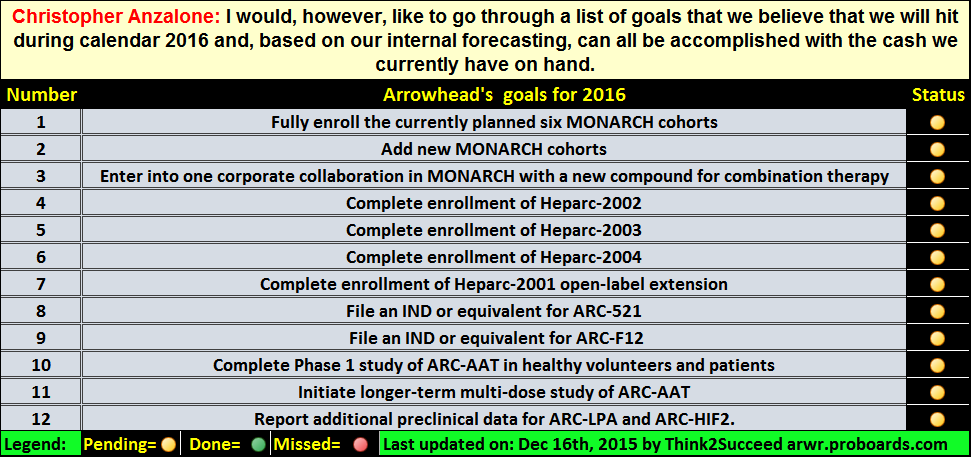
Post by end2war on Dec 1, 2015 12:21:41 GMT -5
Selected summary from Schwab Equity Ratings Report of 12/1/15 about ARWR
11/17/15 Arrowhead Research Corporation presented data at the
AASLD Liver Meeting 2015 in an oral presentation and a poster
showing that ARC-520, its drug candidate against chronic hepatitis B
infection (HBV), leads to robust, sustained anti-viral effects in
chimpanzees with chronic HBV. Arrowhead also describes an
important new discovery that HBV DNA integrated into the host
genome is likely an important source of HBV surface antigen (HBsAg)
production, particularly in chimps that are negative for hepatitis B
e-antigen (HBeAg). The company plans to present additional data
from this study at the 2015 Hep Dart conference in December
demonstrating that two of four HBeAg-positive chimps exhibited
signs of immune reactivation, which is believed to be a necessary
step toward achieving a functional cure of HBV. ARC-520 led to a
dramatic drop in circulating HBsAg, with the degree of HBsAg
reduction correlating with HBeAg status. HBeAg-positive and
-negative chimps demonstrated HBsAg reductions of 1.5 - 2.7 log
(96.8% - 99.8%) and 0.5 - 0.9 log (68.4% - 87.4%), respectively, with
an intermediate response in a chimp transitioning from HBeAg
positive to negative. In addition, one chimp seroconverted for HBeAg
during ARC-520 therapy and had a sustained virologic response with
respect to HBeAg, HBV DNA, and HBsAg. This persisted off therapy
and through at least 32 weeks after ARC-520 and NUC therapy was
removed, which was the last time-point observed. A second chimp
demonstrated effects consistent with immunologic reactivation. In
the study, Arrowhead found that the predominant form of liver HBV
DNA differed in HBeAg-negative versus HBeAg-positive chimps.
Most HBV DNA in HBeAg-positive chimps was cccDNA, while less
than 5% of HBV DNA in HBeAg-negative chimps was cccDNA. In
addition, Arrowhead found that the HBV RNA profiles in
HBeAg-negative chimps were consistent with transcripts arising from
integrated DNA. These data and others, strongly suggest that
integrated DNA is likely an important source of HBsAg production in
HBeAg-negative chimps.
09/28/15 Arrowhead Research Corporation announced top-line
findings from the Heparc-2001 Phase 2a clinical study of ARC-520,
its candidate for the treatment of chronic hepatitis B infection.
Additionally, the company announced findings from a study of 9
chimpanzees that have been treated monthly with ARC-520 for
between 6 and 11 months with a background therapy of
nucleotide/nucleoside analog inhibitors (NUCs) tenofovir and/or
entecavir. Key findings: Arrowhead's proprietary DPC platform can
effectively and consistently knock down target genes in humans HBV
E-antigen positive (HBeAg-positive) patients on a background of
chronic entecavir receiving a 4 mg/kg single-dose of ARC-520
showed a mean maximal 92% (1.2 log) reduction in circulating
HBeAg and a best reduction of 98% (1.7 log). Similar mean maximal
reductions were also demonstrated in HBV core-related antigen
(HBcrAg) from both HBeAg-negative and -positive patients. ARC-520
is designed to silence all gene products expressed by HBV cccDNA,
so this data suggests that it may be substantially disrupting
additional viral functions. ARC-520 achieves significant HBV
s-Antigen (HBsAg) reductions in humans, particularly in treatment
naive, HBeAg-positive patients. In a cohort of NUC-naive,
HBeAg-positive patients, best peak HBsAg reduction has been 99%
(1.9 log) and the mean maximum HBsAg reduction has been 1.05 log
through 15 days post ARC-520 treatment. This open-label cohort is
fully enrolled; data collection is ongoing and will be continued
through Day 85 post ARC-520 treatment. These reductions are
substantially higher than results from NUC treatment-experienced
cohorts. Arrowhead identifies a large target HBV population for
ARC-520 and describes a new paradigm for the HBV lifecycle.
Arrowhead's long-term chimp study and findings from the clinical
study suggest that HBV cccDNA decreases during the HBV lifecycle,
especially with the transition from HBeAg-positive to -negative. HBV
DNA integrated into host DNA appears to maintain significant HBsAg
production as cccDNA declines. This process is accelerated with
NUC treatment. ARC-520 specifically targets cccDNA, and
NUC-naive HBeAg-positive patients are expected to be richest in
cccDNA. It is estimated in the U.S. that 95% of people chronically
infected with HBV are currently NUC-naive and at least 50% of them
are likely to be HBeAg-positive. While it is unknown what impact
ARC-520's broad based effects on HBV biology will have on the
sero-clearance process in any of the HBV subgroups, the effect on
HBsAg in NUC-naive HBeAg-positive patients makes this group
especially attractive to study and a key focus for multi-dose studies
going forward. ARC-520 induces deep HBsAg reduction in chronically
HBV infected chimps and 1 of 4 HBeAg-positive chimps
demonstrated signs of immune reactivation during therapy 9 chimps
were first suppressed with NUCs and then treated with 6 - 11
monthly doses of ARC-520. 4 HBeAg-positive chimps demonstrated
99% (2 log) mean peak reduction in HBsAg, and 1 of the 4
© 2015 Charles Schwab & Co., Inc. (0115-0354) P
11/17/15 Arrowhead Research Corporation presented data at the
AASLD Liver Meeting 2015 in an oral presentation and a poster
showing that ARC-520, its drug candidate against chronic hepatitis B
infection (HBV), leads to robust, sustained anti-viral effects in
chimpanzees with chronic HBV. Arrowhead also describes an
important new discovery that HBV DNA integrated into the host
genome is likely an important source of HBV surface antigen (HBsAg)
production, particularly in chimps that are negative for hepatitis B
e-antigen (HBeAg). The company plans to present additional data
from this study at the 2015 Hep Dart conference in December
demonstrating that two of four HBeAg-positive chimps exhibited
signs of immune reactivation, which is believed to be a necessary
step toward achieving a functional cure of HBV. ARC-520 led to a
dramatic drop in circulating HBsAg, with the degree of HBsAg
reduction correlating with HBeAg status. HBeAg-positive and
-negative chimps demonstrated HBsAg reductions of 1.5 - 2.7 log
(96.8% - 99.8%) and 0.5 - 0.9 log (68.4% - 87.4%), respectively, with
an intermediate response in a chimp transitioning from HBeAg
positive to negative. In addition, one chimp seroconverted for HBeAg
during ARC-520 therapy and had a sustained virologic response with
respect to HBeAg, HBV DNA, and HBsAg. This persisted off therapy
and through at least 32 weeks after ARC-520 and NUC therapy was
removed, which was the last time-point observed. A second chimp
demonstrated effects consistent with immunologic reactivation. In
the study, Arrowhead found that the predominant form of liver HBV
DNA differed in HBeAg-negative versus HBeAg-positive chimps.
Most HBV DNA in HBeAg-positive chimps was cccDNA, while less
than 5% of HBV DNA in HBeAg-negative chimps was cccDNA. In
addition, Arrowhead found that the HBV RNA profiles in
HBeAg-negative chimps were consistent with transcripts arising from
integrated DNA. These data and others, strongly suggest that
integrated DNA is likely an important source of HBsAg production in
HBeAg-negative chimps.
09/28/15 Arrowhead Research Corporation announced top-line
findings from the Heparc-2001 Phase 2a clinical study of ARC-520,
its candidate for the treatment of chronic hepatitis B infection.
Additionally, the company announced findings from a study of 9
chimpanzees that have been treated monthly with ARC-520 for
between 6 and 11 months with a background therapy of
nucleotide/nucleoside analog inhibitors (NUCs) tenofovir and/or
entecavir. Key findings: Arrowhead's proprietary DPC platform can
effectively and consistently knock down target genes in humans HBV
E-antigen positive (HBeAg-positive) patients on a background of
chronic entecavir receiving a 4 mg/kg single-dose of ARC-520
showed a mean maximal 92% (1.2 log) reduction in circulating
HBeAg and a best reduction of 98% (1.7 log). Similar mean maximal
reductions were also demonstrated in HBV core-related antigen
(HBcrAg) from both HBeAg-negative and -positive patients. ARC-520
is designed to silence all gene products expressed by HBV cccDNA,
so this data suggests that it may be substantially disrupting
additional viral functions. ARC-520 achieves significant HBV
s-Antigen (HBsAg) reductions in humans, particularly in treatment
naive, HBeAg-positive patients. In a cohort of NUC-naive,
HBeAg-positive patients, best peak HBsAg reduction has been 99%
(1.9 log) and the mean maximum HBsAg reduction has been 1.05 log
through 15 days post ARC-520 treatment. This open-label cohort is
fully enrolled; data collection is ongoing and will be continued
through Day 85 post ARC-520 treatment. These reductions are
substantially higher than results from NUC treatment-experienced
cohorts. Arrowhead identifies a large target HBV population for
ARC-520 and describes a new paradigm for the HBV lifecycle.
Arrowhead's long-term chimp study and findings from the clinical
study suggest that HBV cccDNA decreases during the HBV lifecycle,
especially with the transition from HBeAg-positive to -negative. HBV
DNA integrated into host DNA appears to maintain significant HBsAg
production as cccDNA declines. This process is accelerated with
NUC treatment. ARC-520 specifically targets cccDNA, and
NUC-naive HBeAg-positive patients are expected to be richest in
cccDNA. It is estimated in the U.S. that 95% of people chronically
infected with HBV are currently NUC-naive and at least 50% of them
are likely to be HBeAg-positive. While it is unknown what impact
ARC-520's broad based effects on HBV biology will have on the
sero-clearance process in any of the HBV subgroups, the effect on
HBsAg in NUC-naive HBeAg-positive patients makes this group
especially attractive to study and a key focus for multi-dose studies
going forward. ARC-520 induces deep HBsAg reduction in chronically
HBV infected chimps and 1 of 4 HBeAg-positive chimps
demonstrated signs of immune reactivation during therapy 9 chimps
were first suppressed with NUCs and then treated with 6 - 11
monthly doses of ARC-520. 4 HBeAg-positive chimps demonstrated
99% (2 log) mean peak reduction in HBsAg, and 1 of the 4
© 2015 Charles Schwab & Co., Inc. (0115-0354) P